Previously we looked at the consequences of metal contamination of the silica used for packing HPLC columns. In this article we’ll consider another aspect of the silica – the nature of the silanol groups.
As I mentioned previously, silica is an amorphous polymer of silicon and oxygen. We think of the surface of a silica particle as terminating in single silanol groups, such as that on the left side of Figure 1. However, work in the early 1990s in Jack Kirkland’s lab at DuPont revealed that this is not necessarily the case. A silicon atom can have two silanol groups attached, as shown for the geminal configuration in the middle of the figure. And if the silanol groups are spaced just right, adjacent silanol groups can share a proton, as shown in the right side of Figure 1.
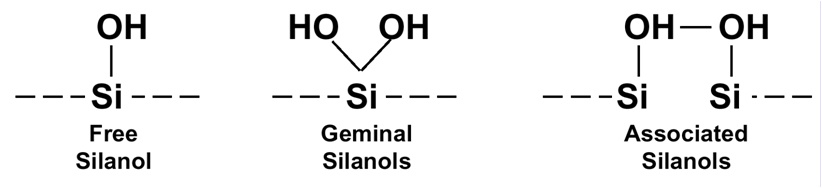
Figure 1
Each of the silanol configurations has different chemical properties that can affect the interactions with solute molecules in an HPLC separation. The solitary silanol groups are acidic relative to the other two configurations. The geminal and associated silanols are less acidic. More-acidic functional groups will, of course, interact more strongly with basic solutes than will their less-acidic counterparts. The tailing of basic compounds has been attributed to higher populations of free silanol groups.
Early silica materials, in addition to the metals discussed last week, had uncontrolled population distributions of the various silanol groups. Batch-to-batch differences in these silanol populations led some users to stockpile columns from one silica batch or even entire silica batches to ensure more uniform performance of columns over time. It was not at all uncommon to purchase a second column of the same part number six months or a year after an initial purchase and find significantly different performance in terms of retention and peak shape.
In addition to reduction of the metal content of silica, manufacturers learned how to treat the silica to shift the population of silanols toward the right in Figure 1, so that the free silanol content was both minimized and consistent. These proprietary techniques are widely used, and are one of the reasons that the newer, high-purity, Type-B silica in use today is so consistent. No longer do we have to worry about changed column characteristics when we purchase a replacement column.
Because the newer silicas have much better performance and are more reproducible, it is prudent to use one of the high-purity silica columns when developing a new method – why start work on the next Nobel Prize with old technology!
This blog article series is produced in collaboration with John Dolan, best known as one of the world’s foremost HPLC troubleshooting authorities. He is also known for his research with Lloyd Snyder, which resulted in more than 100 technical publications and three books. If you have any questions about this article send them to TechTips@sepscience.com




