There are two aspects of buffer lifetime. One has to do with how long it is effective as a buffer and the second is related to other buffer properties – in particular, microbial growth in the buffer solution.
The buffers most commonly used for HPLC with UV detection are phosphate and acetate. As was discussed in ‘Universal Buffer', by combining acetate and phosphate in the same solution, the buffer pH could be adjusted to work anywhere in the normal 2 < pH < 8 region recommended for most reversed-phase columns. Phosphate and acetate, either alone, or in combination, and in solutions of 10 mM or more in concentration should be pH stable for longer than they should be used because of other considerations discussed below.
In contrast, buffers that are used for LC-MS and other evaporative detectors, such as evaporative light scattering (ELSD) or charged aerosol (CAD) may be less stable. This is especially true when carbonate or ammonia are present. These components are sufficiently volatile that there can be a significant loss over a 24-hr period. For this reason, I recommend making such buffers on a daily basis.
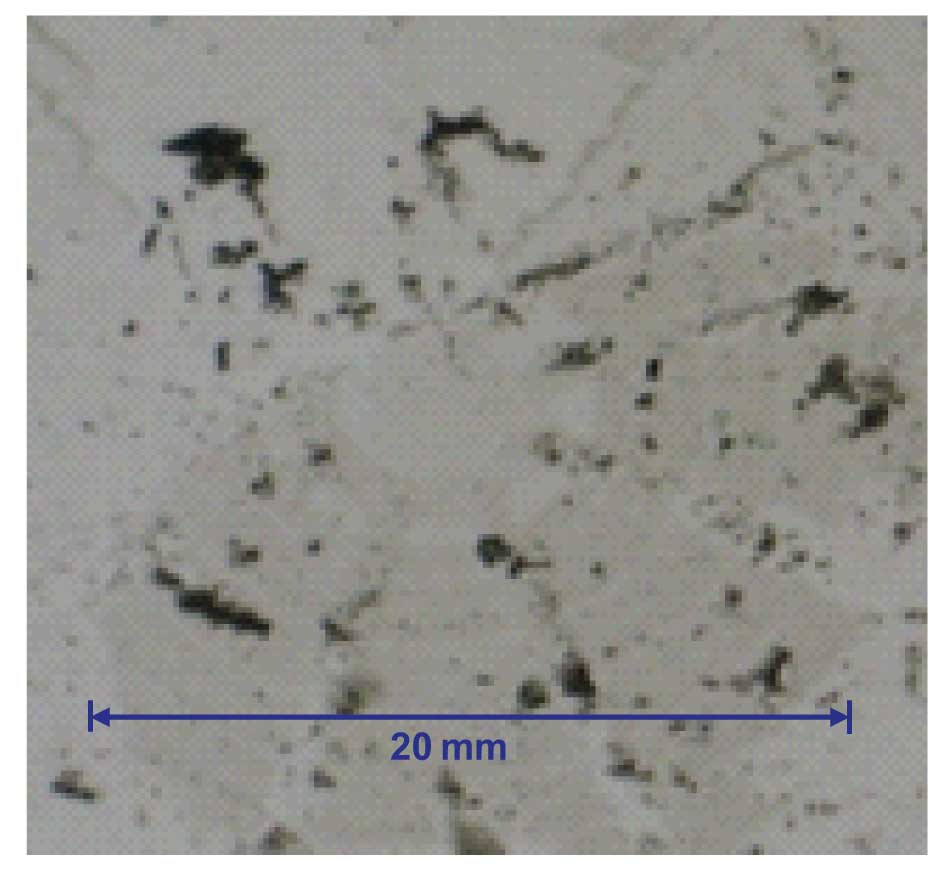 Figure 1
Figure 1
While the buffering capacity and buffer pH can be critical to a separation, there rarely is any damage caused to the HPLC system as a result of buffer deterioration in terms of either of these characteristics. In addition to their chromatographic characteristics, at the concentrations used for HPLC, most buffers offer a very friendly environment for microbial growth. If you leave a buffer standing for long enough at room temperature, it will become cloudy with little creatures growing in it. Such contamination can block frits, coat check valves and generate chromatographic interferences. In the extreme, big chunks of stuff can be present, as is the case shown in Figure 1, where transfer tubing was flushed from the system and the solids collected on a filter. So the big question is how long can you use a buffer without worrying about microbial contamination? As with many questions, the answer varies a bit with the application.
In my experience, most laboratories set a one- to two-week expiration date on buffers without any problem. Years ago we did some testing in our laboratory and found that there was little in the way of microbial contamination in two weeks at room temperature if the buffer container was kept capped. We decided to be conservative, so we wrote our standard operating procedure (SOP) to allow for one-week expiration. I don’t recall any incidents of problems that we could attribute directly to buffers when this limit was observed.
In contrast, my partner Tom Jupille, worked for a company that made single-column ion chromatography columns and equipment. In their hands, they observed sufficient microbial growth in 24 hr to interfere with their analyses. As a result, they made up buffers daily.
As more chromatographers are turning to UHPLC systems, extra caution needs to be taken with buffers. Conventional HPLC systems use 5-µm particle columns fitted with 2-µm porosity frits. Many 3-µm particle columns use 0.5-µm frits, and many workers also use 0.5-µm porosity in-line filters. Most of these frits are tolerant to minor amounts of contamination. UHPLC systems, however, use 0.2-µm frits on the sub-2-µm particle columns, and sometimes elsewhere in the system. This is the same porosity of frit that is used to remove bacteria from solutions, so – you guessed it – they are very effective at filtering out bacteria from the buffer… and getting blocked. For this reason, I recommend making up buffers daily if you are using a UHPLC system.
A couple other precautions will go a long way to avoiding buffer-growth problems. One is to avoid refilling a buffer reservoir. Instead, fill a clean reservoir, then replace it with a new one rather than refilling it when more buffer is needed. If, for some reason, you do observe microbial growth in the buffer, sterilize the system before putting it back in service. Replace the inlet-line frit in the reservoir or it will just inoculate the next batch of mobile phase. It may be necessary to wash the tubing and in-line degasser with a dilute bleach solution (don’t pump this through the column…). Adding sufficient organic solvent to the buffer will also suppress microbial growth. I’ve always considered this to be effective in the 20-30% region, but I’ve also heard of people adding just 5% organic solvent to suppress microbial growth; I don’t have any well-controlled experiments to support either supposition. And the addition of organic may or may not be practical. For example, it will be pretty tough to run a 5-100% acetonitrile-buffer gradient if you have 30% acetonitrile in the buffer!
Better safe than sorry. Keep the buffer expiration period short, regularly clean the reservoirs, and perform system decontamination if contaminated buffer is observed.
This blog article series is produced in collaboration with John Dolan, best known as one of the world’s foremost HPLC troubleshooting authorities. He is also known for his research with Lloyd Snyder, which resulted in more than 100 technical publications and three books. If you have any questions about this article send them to TechTips@sepscience.com




