QuEChERS (Quick, Easy, Cheap, Effective, Rugged, Safe) is a sample preparation technique used primarily in the food industry to extract and clean up samples for instrumental analysis. Originally, the QuEChERS approach was developed for multi-residue pesticide analysis in fruits and vegetables in 2003 to replace more time-consuming and costly sample preparation techniques [1]. Literature shows more than 300 organic contaminants including pesticide residues that can be analysed using QuEChERS as a sample preparation method in one run [2]. Since its emergence many scientists have published a variety of applications not only in fruits and vegetables but also for sample matrices related to biological sample applications [3]. The main reason that QuEChERS has become popular is reflected in its name; the approach is Quick, Easy, Cheap, Effective, Rugged and Safe. Compared with other sample preparation techniques such as the Luke method [4], QuEChERS has clear benefits such as minimized solvent use, simplified steps and no need for special equipment. It is also widely applicable. If you can shake, centrifuge, and pipette, you can prepare samples from fruits, vegetables, meat, dairy products and more for GC or LC analysis. If this sounds attractive you may be asking “Would QuEChERS work for my samples, too?”
Much like buying a car, you have to make sure QuEChERS will fit the needs in your laboratory. If you ask a sales person at the auto dealership “Is this 6 cylinder, 4 door sedan good for me?” they may not be able to provide a useful answer. The selection of a car will bring in many factors such as how many people will be in the car, average commuting distance, safety features, interior room, maintenance costs, warranty, comfort in driving etc. In the world of sample preparation, the same approach should be taken to successfully meet your needs in the laboratory. If the conversation in the auto dealership is translated into the sample preparation world, it will require answers to the following questions. What type of analytes are you investigating? How many people are in the lab? How many samples are prepared and run every day? What is your sample matrix? What instruments and apparatus do you have? What is your budget? What is the current cost related to the overall analytical throughput, including instrument maintenance/repair, labour etc.? The answers to these questions will tell you if QuEChERS is the right choice and how it can be implemented to save time and money.
QuEChERS Protocol
First, let’s review what the QuEChERS protocol is and how it works. The QuEChERS process is mainly composed of three parts. The first step is extraction, which is based on the partitioning of compounds of interest from the aqueous phase into the organic phase (typically acetonitrile) by adding partitioning salts such as magnesium sulfate (MgSO4), sodium acetate (NaOAc), sodium chloride (NaCl) etc. Its mechanism is analogous to conventional liquid-liquid extraction but instead it uses acetonitrile to replace water-immiscible and environmentally hazardous solvents such as methylene chloride. Since acetonitrile is water-miscible, the phase separation between the aqueous and the acetonitrile layers is facilitated with a mixture of partitioning salts. A typical example of the QuEChERS extraction step starts with weighing 10 g of fruits or vegetable in a 50 mL tube followed by the addition of 10 mL acetonitrile and partitioning salts. Then one minute of hand or mechanical shaking and a few minutes of centrifugation will give phase separation as shown in Figure 1. The main purpose of the extraction step is to pull the analytes from the sample matrix into the upper acetonitrile layer. The extraction step is effective but not selective, resulting in the transfer of analytes along with some of the unwanted matrix components to the acetonitrile layer. To get rid of the unwanted matrix components while keeping your compounds of interest in the upper acetonitrile layer, the second step of the QuEChERS procedure implements a ‘clean up’ step by dispersive solid-phase extraction (dSPE). Unlike conventional packed SPE in a cartridge or well plate, dSPE is ‘loosely’ packed in either a 2 mL or 15 mL plastic centrifuge tube. You transfer the upper acetonitrile layer from the 50 mL extraction tube to the tube containing dSPE adsorbent materials. Depending on your sample matrix and compounds of interest, different dSPE sorbents are available in pre‑weighed packets and bulk containers. After the transfer of the upper acetonitrile layer to the dSPE tube, several seconds of vortex mixing and a few minutes of centrifugation will form a hard pellet at the bottom of the tube. In the third step, the supernatant can be directly injected, diluted with mobile phase, or evaporated and reconstituted in an appropriate solvent for LC/MS/MS or GC/MS/MS analysis or both. No special SPE manifold, special glassware or halogenated solvents are used during the entire sample preparation. The whole sample preparation takes 10 to 20 min and you can make multiple samples simultaneously.
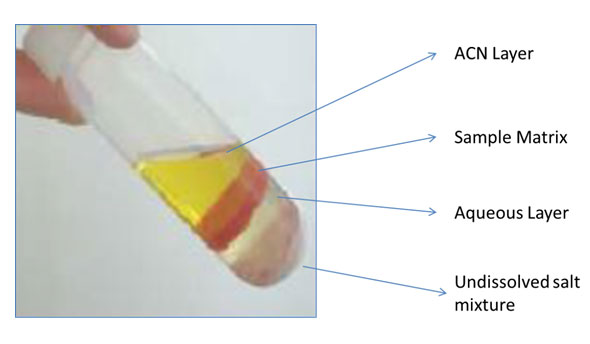
Figure 1: Phase separation between acetonitrile and the aqueous layers in chicken after centrifugation in the QuEChERS extraction step.
Now that you understand how QuEChERS works, let’s take a look at its method development approach and optimization. Method development can be easier using QuEChERS pre-weighed packets containing extraction salts (e.g., Extraction kit with 6 g MgSO4 and 1.5 g NaOAc with 50 mL centrifuge tube) and dSPE cleanup tubes (e.g., 150 mg MgSO4, 50 mg PSA and 50 mg C18 in 2 mL tube). There are a handful of QuEChERS extraction kits and dSPE kits to choose from and if you’d like to optimize QuEChERS further, you can use bulk materials and manually add C18, PSA, MgSO4 etc. This three-step QuEChERS protocol is outlined in Figure 2.
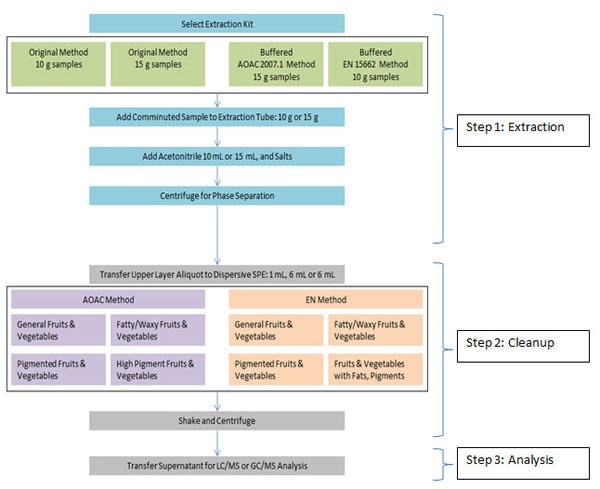
Figure 2: Generic QuEChERS method protocol highlighting step 1: extraction, step 2: dSPE clean up and step 3: analysis by LC/MS and GC/MS.
The easiest and simplest route to take in method development is to screen a combination of commercially available pre-weighed QuEChERS kits in a ‘mix-and-match’ fashion as shown in Figure 3. To calculate the number of samples required for your method optimization, use the following formula: number of extraction kits × number of dSPE types for clean up = total number of samples. In this example, if you chose to use the EN and AOAC extraction kits with four different dSPE tubes for each extraction there are a total of eight samples, which you can prepare within 10 to 20 min. If your analytical run time is 20 min, then you’d need 160 min to complete your analysis. In about three hours of lab time you will get an idea of which combination of extraction salts and dSPE sorbents will perform the best by comparing the chromatograms from the eight samples. Once the right combination of extraction and dSPE kits are chosen you can modify your method even further to improve the results if you desire. In the hypothetical example shown in Figure 3, buffered EN extraction and general fruits and vegetables dSPE give the best chromatographic signal responses and would, therefore, be the best combination to use.
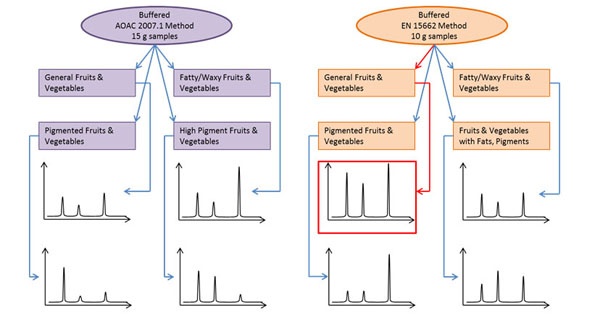
Figure 3: Method development diagram depicting two extraction kits and four dispersive clean-up kits generating eight chromatograms for comparison.
Tips and Tricks in the Optimization of QuEChERS Applications
There are numerous QuEChERS guidelines and applications published in the form of application notes, journal articles and official methods such as CEN (European Committee for Standardization) and AOAC International [5–6]. For general sample matrices such as fruits and vegetables, sea food and meat, following the ‘mix-and-match’ initial screening method described in the previous section will give your laboratory the information it needs to determine if the QuEChERS approach is the sample preparation of choice. However, you may encounter difficulties testing some challenging sample matrices. Variables to consider in QuEChERS optimization are given in Figure 4.
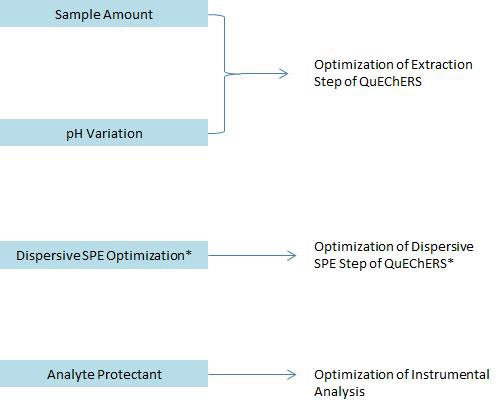
Figure 4: Variables to consider in QuEChERS method optimization (*Dispersive SPE optimization using bulk sorbent materials is optional and is not discussed here.)
The first parameter to consider is the amount of the sample being used. Many QuEChERS applications have fixed amounts of samples in the extraction step depending on which guidelines they are based (CEN QuEChERS applications use 10 g of sample and AOAC International QuEChERS applications use 15 g of sample). A fixed amount of sample is necessary due to the sample types tested in the earlier years of QuEChERS development. The majority of the samples were fruits and vegetables for pesticide detection. Fruits and vegetable contain a large portion of water and as previously discussed, the first step of QuEChERS is extraction in a 50 mL tube with acetonitrile and partitioning salts added to separate the aqueous and acetonitrile layers. The aqueous layer content comes from the sample itself due to the high moisture content in fruits and vegetables. Today QuEChERS is used not just for fruits and vegetables and when you test samples that do not contain adequate moisture, effective phase separation may not occur between the aqueous and acetonitrile phases when a fixed sample amount is used. These types of samples can include juice concentrates, fish, dried leaf, grains, nuts, meat etc. In this case you can use less than the suggested amount of sample and add enough water to bring the total sample loading to 10 or 15 g. For example, if you are following an AOAC official QuEChERS protocol, you may add 2 g of sample followed by 13 mL of water to make a 15 g sample in the 50 mL extraction tube [7]. Testing 2, 5, and 7 g of sample will require 13, 10, and 8 g of water, respectively, and subsequent analysis of the analyte peak area and signal-to-noise in the chromatograms will give you an idea of how much sample is optimal for your QuEChERS application. Juice concentrate samples with unknown concentration factors are very good examples of this optimization process [8]. You may obtain the °Bx information to find the right amount of sample from the CEN method [9], but the °Bx value may not be very helpful if the texture of your juice concentrate is very thick, with an unknown concentration factor.
Another consideration is pH variation. Sometimes you may not have enough information for your sample. Let’s revisit the case of juice concentrate samples with an unknown concentration factor. You may need more information for the juice concentrates, such as whether they contain peel from the fruit when they were prepared, what the concentration factor is, and what the pH level is. If it is too concentrated and the texture of the juice concentrate is extremely thick, it will not be easy to measure the correct pH level of the sample. For base-sensitive pesticides there have been many trials to improve the recovery of these analytes [10]. On the other hand, if your sample matrix is extremely acidic, your compounds of interest may not be extracted efficiently and your results may not meet the acceptance criteria set for validation. Once you select the sample amount mentioned above, use that amount and test different pH levels during the extraction. In the case of juice concentrates, you may add 0, 0.6, 1, and 2 mL of 5 N NaOH to give different pH environments and determine any enhancement in method performance. Some compounds show dramatic improvements, as seen in Figure 5.
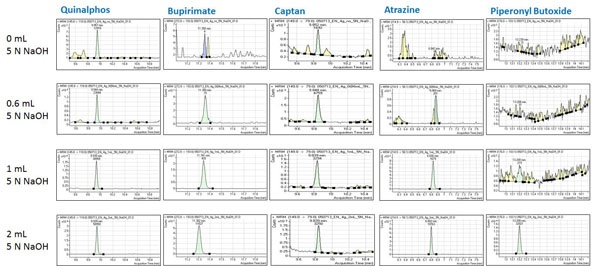
Figure 5: The improvement of sensitivity with pH variation in the QuEChERS extraction step for quinalphos, bupirimate, captan, atrazine and piperonyl butoxide.
Another consideration is the use of analyte protectants (AP). APs can play an important role when the analysis is done using a GC system. Many publications are available using different compounds as APs [11]. Usually these compounds are very affordable and easy to find from many chemical companies. A mixture of D-sorbitol and L-gulonolactone can serve as successful APs [12]. Some GC columns can have active sites resulting in analyte interactions that produce poor peak shapes and reduced sensitivity. Newer GC systems and GC columns are a lot better in terms of inertness with very limited or no active sites but can still acquire them over time as matrix components deposit. QuEChERS is not designed to make extremely clean samples, but rather to accommodate a large list of compounds, up to 375 [2] in various sample matrices. Therefore, even if the GC system, GC columns and other instrument components in the GC flow path were inert in the beginning of your project, it is very likely that you will make part of the analytical system non-inert. Some of the extreme cases show the difference between GC/MS/MS runs with and without the APs (Figure 6).
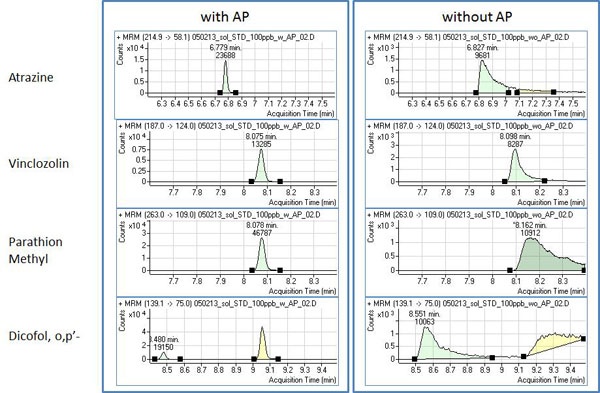
Figure 6: Chromatograms demonstrating the performance differences for analytes using GC/MS/MS with and without APs in lemon juice concentrate.
Make the Simple the Simplest
One of the great benefits offered by QuEChERS is that it is simple to use. Without the need for special equipment, supplies and accessories (e.g., vacuum manifolds, positive pressure devices, environmentally hazardous solvents, special glassware etc.), you can prepare your samples for GC or LC analysis. Unlike cartridge or plate-based sample preparation products such as SPE, there are no flow-rate steps during the entire QuEChERS procedure. Pipetting technique and centrifugation is all that is needed to prepare samples from a variety of matrices. There are a handful of different QuEChERS products available with slight variations in the extraction salts and sorbent types to choose from and by following the tips and tricks provided in this article and testing some pre-weighed QuEChERS products on your samples, you will have your QuEChERS method optimized within a day or so, providing fast, reliable and reproducible results for your laboratory.
References
1. Michelangelo Anastassiades and Steven J. Lehotay, J. AOAC Int., 86 (2003), 412 – 431
2. Kaushik Banerjee et al, J. Chrom A, 1270 (2012), 283-295
3. Nuria Leon et al, J. Chrom A, 1258 (2012) 55-65
4. Luke et al, J. AOAC Int., 68(1) (1985), 64-71
5. Method Validation and Quality Control Procedures for Pesticide Residues Analysis in Food and Feed, SANCO/12495/2011
6. AOAC Official Method 2007.01, Pesticide Residues in Foods by Acetonitrile Extraction and Partitioning with Magnesium Sulfate
7. Agilent Technologies, Inc. Application Note, Modified QuEChERS for HILIC LC/MS/MS Analysis of Nicotine and Its Metabolites in Fish (5991-2408EN)
8. North American Chemical Residue Workshop poster (2013), A Modified QuEChERS Approach for the Analysis of Pesticides in Fruit Juice Concentrate
9. Foods of Plant Origin – Determination of Pesticide Residues Using GC-MS and/or LC-MS/ MS Following Acetonitrile Extraction/Partitioning and Clean-up by Dispersive SPE (QuEChERS- method). EN 15662:2008
10.Steven J. Lehotay et al, J. AOAC Int., 88(2) 2005, 615 – 629
11.Mateřina Maštovská et al, Anal. Chem., 77 (2005), 8129 – 8137
12.Michelangelo Anastassiades et al, J. Chrom A, 1015 (2003) 163 – 184




