This application note describes a method for the analysis of Class 2 solvents (water-soluble samples) that are unsuited to Headspace GC methods.
 The determination of residual solvents is crucial for the safety and quality of pharmaceuticals. The United States Pharmacopeia (USP) has classified these solvents into three main classes – Class 1, 2 and 3. Headspace gas chromatography testing methods have been employed for the determination of Class 1 and 2 residual solvents. However, there is no mention of methods for sub-group Class 2C solvents, which have higher boiling points and are not suited for headspace analysis.
The determination of residual solvents is crucial for the safety and quality of pharmaceuticals. The United States Pharmacopeia (USP) has classified these solvents into three main classes – Class 1, 2 and 3. Headspace gas chromatography testing methods have been employed for the determination of Class 1 and 2 residual solvents. However, there is no mention of methods for sub-group Class 2C solvents, which have higher boiling points and are not suited for headspace analysis.
In this application note, we describe a direct liquid injection method for the analysis of Class 2C residual solvents using the Nexis™ GC-2030 gas chromatograph. The results achieved resolution equivalent to residual solvents of other Class 2 solvents. Furthermore, the method demonstrated high repeatability in both Class 2C standard solutions (%RSD between 0.99 and 3.68) and a sample (headache medication) solution (%RSD between 0.29 and 4.00). The method also showed favourable recovery rate between 91% and 107%. This described direct liquid injection method proved to be suitable for the gas chromatographic analysis of Class 2C residual solvents in pharmaceuticals.
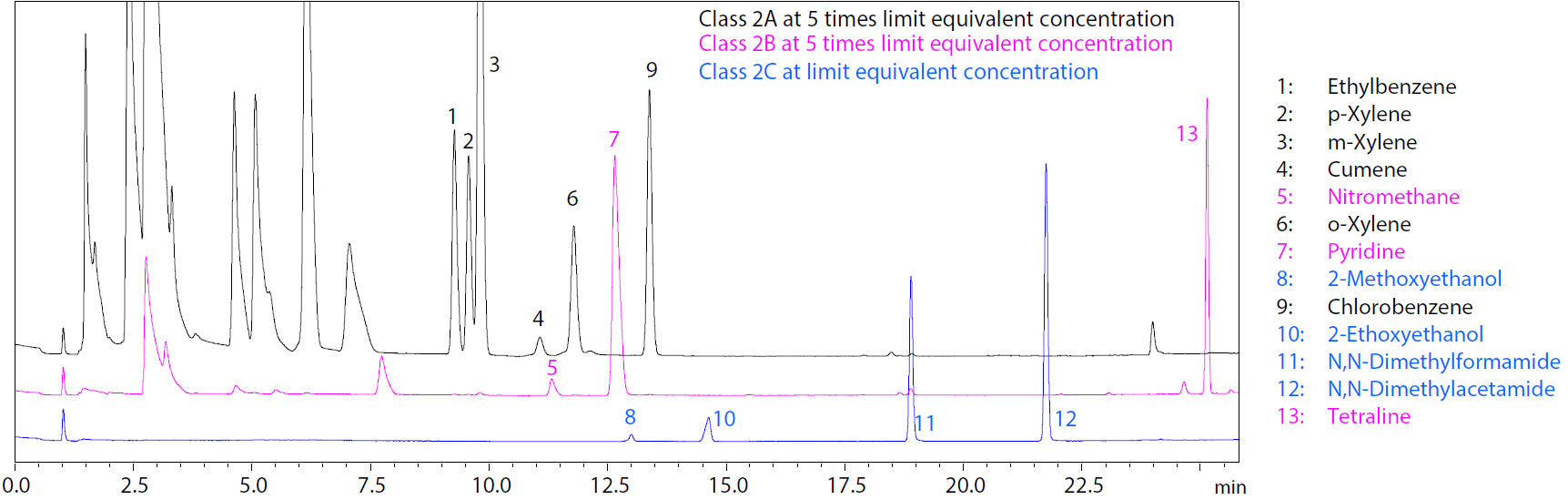
Superimposed Chromatograms of Class 2C Standard Solution and Class 2A and 2B Standard Solutions.
Download this application note and...
- Explore the use of Nexis™ GC-2030 for the direct liquid injection analysis of Class 2C solvents in pharmaceuticals
- Compare the separation and analysis of Class 2A, 2B and 2C solvents using the same analytical method, conditions and gas chromatograph.




