Shimadzu has produced an application note demonstrating how the LC-MS/MS bioanalysis of antibody drugs has been significantly simplified by this unique nSMOL technology. In this application, the analysis of Trastuzumab is discussed.
 Introduction
Introduction
One of the greatest benefits of antibody drugs is the safety as this same molecule constitutes our natural defence system against antigens. However, this benefit turns into a barrier to efficient bioanalysis; methodology is needed to selectively detect administered antibody without interference from host-derived antibodies. Although LC-MS/MS is expected to be the alternative to the conventional ELISA technique for its robustness and ease of method development, strong ionization suppression exerted by high-abundance peptides derived from the constant (Fc) region limits the sensitivity of low-abundance peptides in the variable (Fab) region.
 What is nSMOL technology?
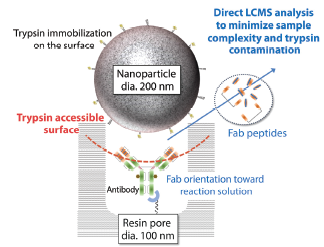
What is nSMOL technology?
Nano-surface and molecular orientation limited proteolysis (nSMOL™) is Shimadzu’s proprietary, innovative technology that enables selective proteolysis of the Fab region of antibodies. Peptide sample prepared by the nSMOL kit has a substantially lower complexity near-zero interference from host antibodies, thus enabling LC-MS/MS sensitivity to reach down to the level required for pre-clinical and clinical studies. This application news reports the fully-validated LC-MS/MS method developed for Trastuzumab determination in plasma.
By downloading this application note you will learn:
- how LC-MS/MS bioanalysis of antibody drugs can be significantly simplified by this unique nSMOL technology




