Shimadzu has produced a poster detailing how data integrity compliance can effectively be achieved on a unified network platform in the analytical laboratory.
Data integrity compliance has become one of the most important components of industry’s responsibility to ensure the safety, efficiency and quality of drugs to protect human health. However, regulatory agencies have increasingly observed Current Good Manufacturing Practice (CGMP) violations related to data integrity during inspections in recent years. This is because not only the role of data integrity on the CGMP requirements is not well understood, but the scope of data integrity is more than earlier expectations in the analytical laboratory. Regulatory agencies expect that data must be reliable and accurate on the CGMP criteria for all laboratory instruments, including UV-Vis and FT-IR, as well as all other standalone instruments to the same level of chromatographs. Here, it is shown how data integrity compliance is effectively achieved on a unified network platform in the analytical laboratory.
 Topics covered in this poster include:
Topics covered in this poster include:
- Guidance for Data Integrity Issued by Regulatory Agencies
- Ensuring Data Integrity Compliance
- Example of FDA Warning Letter
- Actual Situation in the Regulated Laboratory
- Issues to be Solved
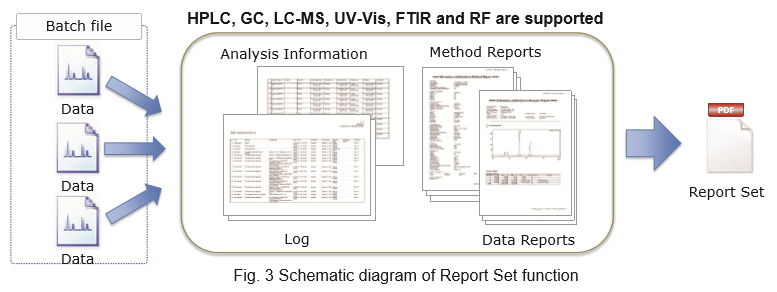
- Report Set Function to Support Data Integrity
- Features of Report Set Function
- Conclusion
View the on-demand webinar on 'Assuring Laboratory Data Integrity in a Time of Enhanced Regulatory Oversight' by David Stokes (Principal Consultant and Director, Convalido Consulting Ltd, Newcastle-Upon-Tyne, UK) and be able to download the poster.




