A reader wrote me recently asking what would happen if he injected his sample, which came dissolved in hexane, into a reversed-phase mobile phase of methanol-buffer. The first answer is that this is not a good habit, but is it possible? We’ll see.
Effects of Strong Organic Solvents on Peak Shape
The use of strong organic solvents can significantly impact peak shape, often leading to peak distortion that can hinder accurate analysis and interpretation.
Peak Distortion from Strong Solvents
When a large sample is injected in a solvent stronger than the mobile phase, peak distortion can occur. An example of this is shown in Figure 1, where a mobile phase of 18% acetonitrile (ACN) is used. On the right, in Figure 1b, a 30 µL injection of sample is made using mobile phase as the injection solvent and sharp peaks are observed. If, on the other hand, the sample is dissolved in 100% ACN, as is the case on the left, in Figure 1a, we observe two things. We observe that the peaks are distorted, especially the first one, and a closer look shows that the retention times are shorter than normal. If you think about what is happening in the column, this makes sense. The strong injection solvent enters the column and must get mixed and diluted with the mobile phase.
Understanding Retention Time Shifts
If the volume is large, as in the Figure 1a, the plug of solvent travels down the column at the flow rate, becoming diluted as it travels. Meanwhile, sample molecules on the outside of the injection plug “see” the true mobile phase and act normally, whereas the molecules in the middle of the plug “see” the injection solvent as the mobile phase and travel faster than normal. The result is a sample that gets smeared along the column until the injection solvent gets diluted out. And once smeared onto the column, the sample doesn’t recover, so distorted peaks appear. Because some of the molecules are flushed quickly down the column during the dilution process, the average retention time is shorter.
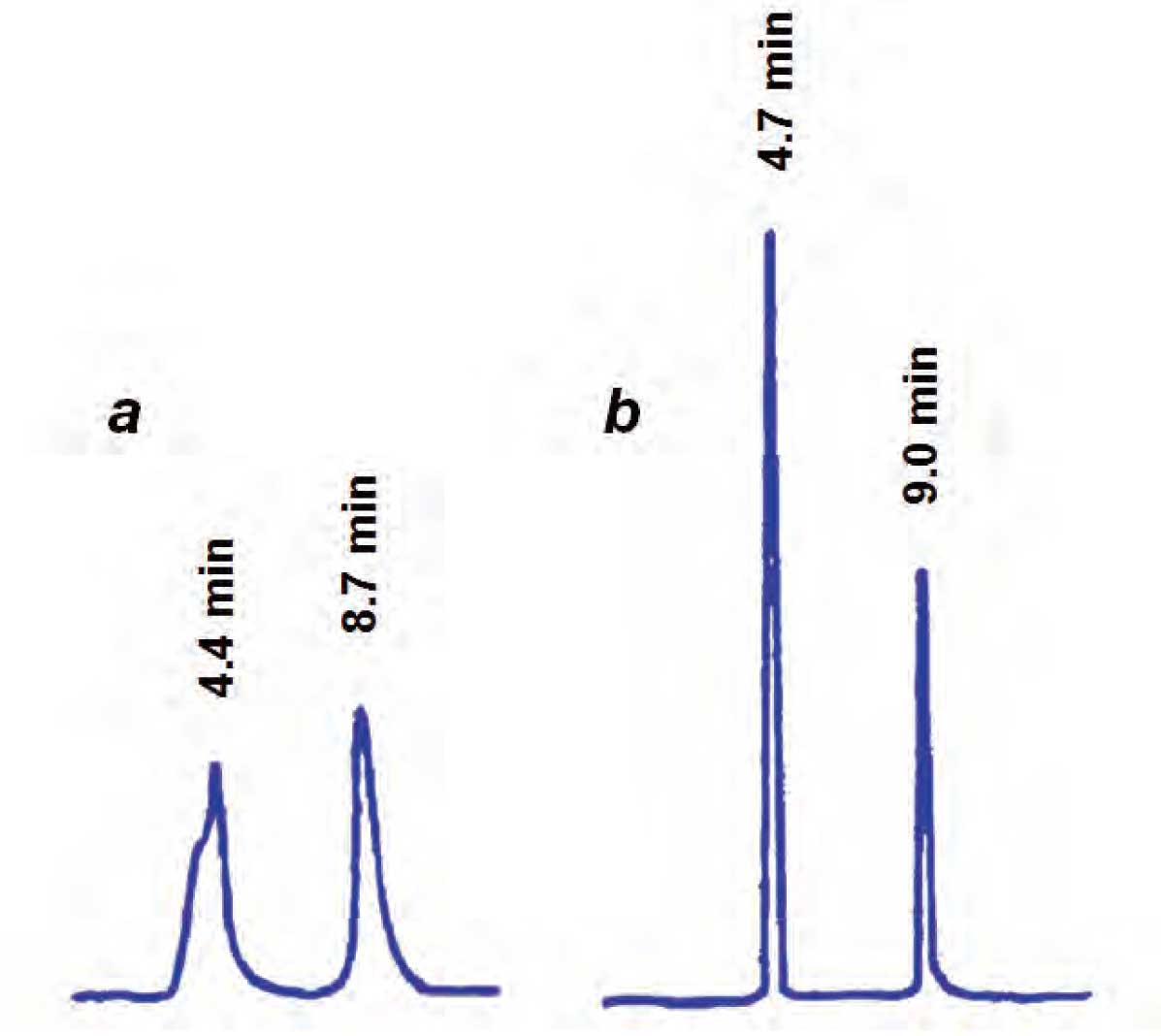 Figure 1
Figure 1
Strategies for Using Strong Solvents
When working with strong solvents, implementing specific strategies can improve peak quality.
Reducing Injection Volume for Optimal Results
The fix to the problem of Figure 1a should be obvious. Inject in a weaker solvent or inject in such a small volume that it is equilibrated instantaneously with the mobile phase or both. As a rule of thumb, we can inject approximately 15% of the volume of the first peak of interest if we inject in mobile phase. For example, if the first peak is 0.1 min wide at the base and the flow rate is 1 mL/min, the peak volume is 100 µL, so a 15-µL injection in mobile phase should be possible. If stronger injection solvents are used, smaller volumes must be used.
Benefits of Weaker Injection Solvents
Weaker injection solvents allow larger injections, and if the injection solvent is >20% lower concentration than the mobile phase (e.g., a 30%B injection solvent and 50%B mobile phase), very large injections can be made, taking advantage of a process called on-column compression.
Experimenting with Non-Miscible Solvents
So where does this leave us with hexane as an injection solvent? Not only is the injection solvent much stronger than the mobile phase, but it is not miscible either. This sounds like a disaster waiting to happen – and it may be. But not so fast! If the injection volume is small enough, it may be possible to disperse the injection solvent in the mobile phase sufficiently that acceptable peak shape results. This will be a matter of trial and error. I would start by making a solution of my sample in hexane at a high enough concentration that I get a good response, even at very small volumes. First, inject the sample dissolved in mobile phase as a reference. Then inject 1 µL, 2 µL, 5 µL, 10 µL, and 20 µL of the hexane solution and see what happens. I expect that 1 µL will be acceptable, but at some volume, the peaks will start coming out too early and will be distorted, as in Figure 1a. No, it’s not ideal, but it may work. I remember doing exactly this with a sample we received that was dissolved in toluene. We already had a method for the same compound as a reversed-phase method. If I recall correctly, we were able to run with 5-µL injections and obtain acceptable results.
Conclusion: Balancing Theory with Experiment
My favorite chemistry quote is from Izaak Kolthoff, considered by many to be the father of analytical chemistry: “Theory guides, experiment decides.” Here is a good example of that – no, hexane is not compatible with a methanol-water mobile phase, but under the right conditions, you just might get away with it.
This blog article series is produced in collaboration with John Dolan, best known as one of the world’s foremost HPLC troubleshooting authorities. He is also known for his research with Lloyd Snyder, which resulted in more than 100 technical publications and three books.




