The sample solvent, also called dissolution solvent or injection solvent, is the solvent used to prepare the solution in which the compound to be analysed by HPLC is dissolved. The choice of the sample solvent is fundamental for a successful separation, since this determines the initial retention of the compound on the stationary phase. Sample solvents with stronger elution strength than the initial conditions of the mobile phase cause a less effective retention with undesirable consequences, such as peak distortion, peak broadening and earlier elution.
It is good practice in the development of a HPLC method to dissolve the sample in the initial mobile phase or in a miscible weaker solvent. This is even more important in HILIC separation as it is generally more sensitive to the solvent strength mismatch between the sample solvent and the mobile phase in comparison to reversed-phase chromatography.
In HILIC, aqueous sample solvents have a high elution strength, and this can impede the partitioning of the analytes into the stationary phase. This effects predominantly the earlier eluting highly polar analytes, for which substantial peak distortion and decreased retention can be observed. Therefore, the lowest possible amount of water should be used for the preparation of the sample solvent, ideally pure acetonitrile.
However, this can cause solubility issues with highly polar analytes. A good approach is to prepare stock solutions in higher amounts of water. Typically water/acetonitrile (50:50, v/v ) are suitable for most applications. Then, working solutions can be prepared by dilution in the mobile phase, which usually contains 80–95% (v/v ) acetonitrile or in pure acetonitrile. If the solubility is still problematic with a high content of acetonitrile, working solutions may be prepared in mixtures of acetonitrile/isopropanol (50:50, v/v ).
It is important to remember that for methods including a needle wash step, the wash-solvent should also contain at least 80–90% (v/v ) acetonitrile, since part of the wash-solvent can be injected with the sample.
An alternative solution that can overcome the negative effect of solvent strength mismatch between the sample solvent and the mobile phase is the use of the minimum sample volume. In the forthcoming instalment, we will move our focus from the pure chromatography and we will discuss the combination of HILIC separation with MS detection.
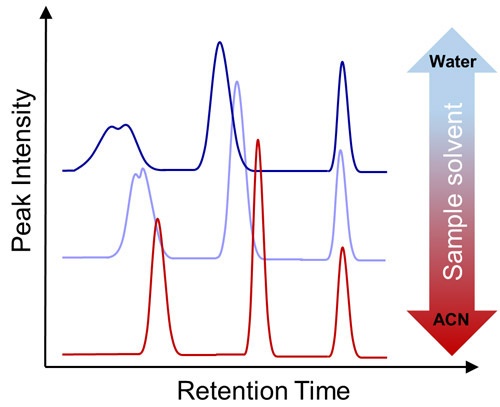
Figure 1: The effect of sample solvent in HILIC separation: dissolving the sample under pure acetonitrile gives the best results. Increasing the water content in the sample solvent causes peak distortion, peak broadening and earlier elution of the more hydrophilic compounds at lower retention times.
This blog article series is produced in collaboration with Dr Giorgia Greco, Product Manager with Thermo Fisher Scientific in Germany and Thomas Letzel, Associate Professor and Head of the Analytical Research Group at the Technische Universität München, Germany.
Giorgia Greco received a PhD in Chemistry and worked as a Post Doc researcher at the Technische Universität München, Germany. During her research, she specialized in the fundamental of LC-MS and in the separation and analysis of metabolites from human and food matrices, as well as organic contaminants in waste water samples, by hyphenated HPLC/MS and HILIC/MS techniques.
Thomas Letzel received his PhD in Chemistry with Aerosol Analysis and then worked as a Post-Doc performing pharmaceutical analysis. He is the author of more than 50 publications and two books and wants to share his experience in liquid chromatography, especially in HILIC, with the community to accelerate the dissemination about HILIC theory and practical handling.
If you have any questions about this article send them to techtips@sepscience.com




