Are your analytes eluting close to the void volume of a reversed-phase (RP) column? Do you have problems retaining organic molecules under conventional reversed‑phase liquid chromatography (RPLC) conditions? Have you increased the initial percentage of water in the mobile phase or tried a different mobile phase (and pH) or changed to a polar-embedded RP column without success? What do you do? Have you tried HILIC?
Hydrophilic interaction liquid chromatography, referred to as HILIC, has been known for several years as a ‘non-robust’, ‘difficult’ separation technique with long equilibration times. This statement has often been announced by analysts originally experienced in RPLC. However, this is not true if you are careful about several key aspects.
We will give you several important hints via this tip series, thus providing you with the basis of developing ‘robust’ and ‘handy’ HILIC methods.
Indeed HILIC is a very powerful new tool for the separation of polar compounds poorly retained under RPLC conditions with roots in the 1970s. It is also known as ‘reversed-RPLC’ because the mobile phase is a mixture of water/water-miscible organic solvent as with RPLC but solvent elution strengths are opposite. In gradient mode, HILIC starting conditions require a high percentage of organic solvent, typically 95%, and the elution is promoted by increasing the water content in the mobile phase up to 40%. The HILIC stationary phases are very polar materials such as silica or polar-bonded silica-based phases, whose main characteristic is to strongly adsorb water on their surface.
Hydrophilic compounds are highly retained in HILIC due to a favoured solubilization into the adsorbed water layer, whereas hydrophobic compounds are eluted earlier.
Therefore, the inversion of the elution order is quite a common experience when HILIC columns are compared with RPLC columns.
Let us consider two analytes with different polarity: toluene and 4-hydroxybenzoic acid. The first is very hydrophobic while the second is hydrophilic. As shown in Figure 1, toluene is strongly retained, while
4-hydroxybenzoic acid is not retained by a conventional C18 RP column. Moreover, when the two analytes are injected on a HILIC diol phase, the elution order is reversed as 4-hydroxybenoic is retained longer.
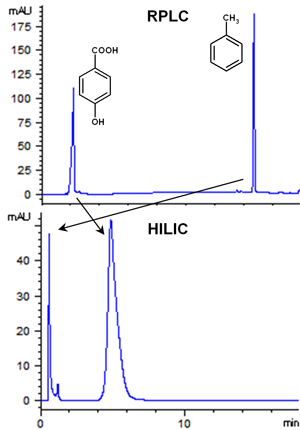
Figure 1: Separation of 4-hydroxybenzoic acid and toluene on RPLC and HILIC. Chromatographic conditions: RPLC: column, Zorbax ECLIPSE XDB-C18 (150 x 4.6 mm, 5 µm; mobile phase, water/ACN,
0-15 min, from 20 to 90% ACN; flow-rate: 0.6 mL/min; UV detection: 250 nm. HILIC: column, YMC-Diol (150 x 2.1 mm, 5 µm); mobile phase, ACN/water (95/5, v/v), 10 mM ammonium acetate; flow-rate:
0.6 mL/min; UV detection: 250 nm.
The separation of small polar compounds such as organic acids, basic amines, and water soluble vitamins, that can be difficult on a C18 column, can easily be performed in HILIC.
But how do we can decide between RPLC and HILIC?
Analyte polarity is the first parameter to be considered when we have to select the right HPLC method. Both RPLC and HILIC retention mechanisms are based on a partitioning process. Even if interactions of different nature may contribute to the separation, from a practical point of view analyte polarity can be well described by the logarithm of the partition coefficient, log P,
log Poct/wat = log ([analyte]oct/[analyte]wat)
where P is defined as the ratio of concentrations of an unionized analyte in n-octanol and water phases at equilibrium. Clearly, hydrophobic compounds are preferentially solubilized in the n-octanol phase and hydrophilic ones in water (Figure 2).
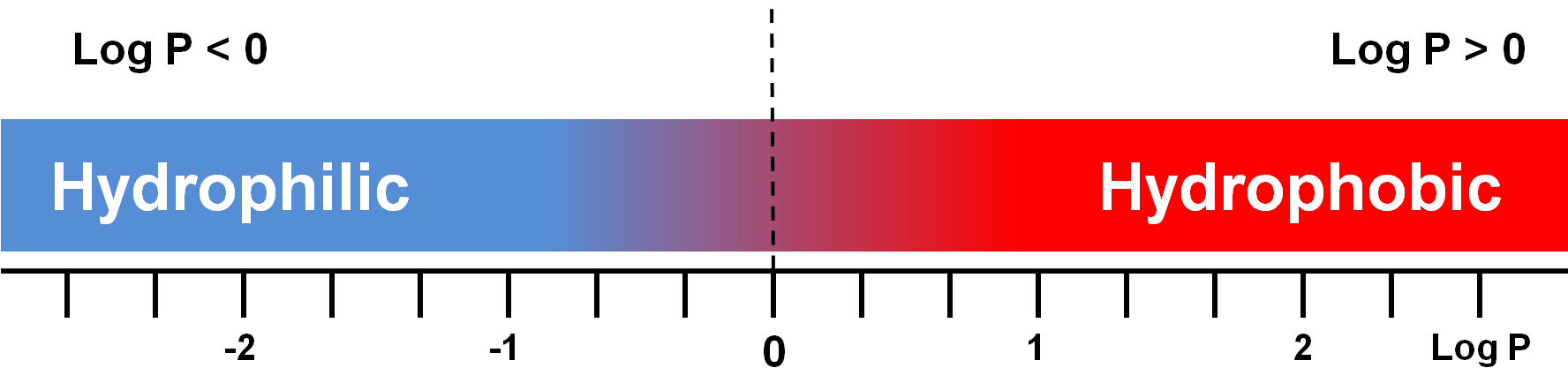
Figure 2: Graphical representation of log P values in relation to analyte polarity. Hydrophilicity increases with the decreasing of log P value.
As a consequence in the case of ionizable analytes, such as amine or organic acids, the polarity changes with the pH. Charged analytes are more hydrophilic than their neutral form and for them the logarithm of the distribution coefficient, log D, should be considered instead of log P,
log Doct/wat = log ([analyte]oct/[ charged analyte +neutral analyte]wat)
where D is the ratio between the concentration of the analyte in n-octanol and the sum of the concentrations of the charged and neural form in water at a given pH (Figure 3).
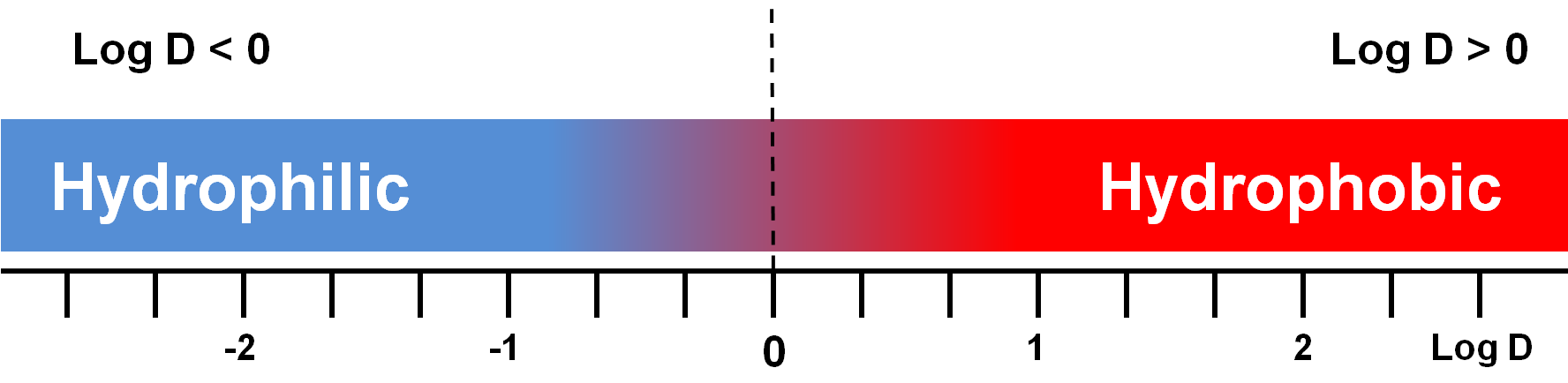
Figure 3: Graphical representation of log D values in relation to analyte polarity. Hydrophilicity
increases with the decreasing of log D value.
In contrast to log P, log D is pH dependent and the pH at which it has been measured has to be always specified. Similarly to log P, log D can be measured experimentally. However, such a procedure is quite long and would not be compatible with routine analyses. A number of different software packages for a log P and a log D calculation can assist us for a rapid evaluation of analytes polarity.
Among them, www.chemicalize.org and www.chemspider.com are free databases that contain log P and log D values predicted with Marvin and ACD/PhysChem Suite software, respectively.
Let us consider again toluene and 4-hydroxybenzoic acid. The mobile phase pH is neutral in both conditions presented in Figure 1.
Keep in mind that in case of unionizable compounds, such as toluene, log P = log D.
In order to select the most appropriate HPLC separation mode, you can follow this scheme:
log P or log D > 0 → RPLC
log P or log D < 0 → HILIC
Now look at the polarity of your analytes. If they are hydrophilic, they are efficiently separated in HILIC.
In the next instalments, we will present the basis of HILIC, the main stationary phases commercially available and how to select the chromatographic parameters (i.e. organic solvent, salt content, mobile phase pH, and column temperature) for your best performance.
This blog article series is produced in collaboration with Dr Giorgia Greco, Product Manager with Thermo Fisher Scientific in Germany and Thomas Letzel, Associate Professor and Head of the Analytical Research Group at the Technische Universität München, Germany.
Giorgia Greco received a PhD in Chemistry and worked as a Post Doc researcher at the Technische Universität München, Germany. During her research, she specialized in the fundamental of LC-MS and in the separation and analysis of metabolites from human and food matrices, as well as organic contaminants in waste water samples, by hyphenated HPLC/MS and HILIC/MS techniques.
Thomas Letzel received his PhD in Chemistry with Aerosol Analysis and then worked as a Post-Doc performing pharmaceutical analysis. He is the author of more than 50 publications and two books and wants to share his experience in liquid chromatography, especially in HILIC, with the community to accelerate the dissemination about HILIC theory and practical handling.
If you have any questions about this article send them to techtips@sepscience.com




