In Part 3 we bring together the retention mechanism concepts discussed earlier to form a simple view of the nature of selectivity in liquid stationary phases used in gas chromatography.
Retention in gas chromatography, as discussed earlier, depends on the total interactions, polar + apolar, between a solute and a stationary phase; retention is a function of the sum of all possible interactions.
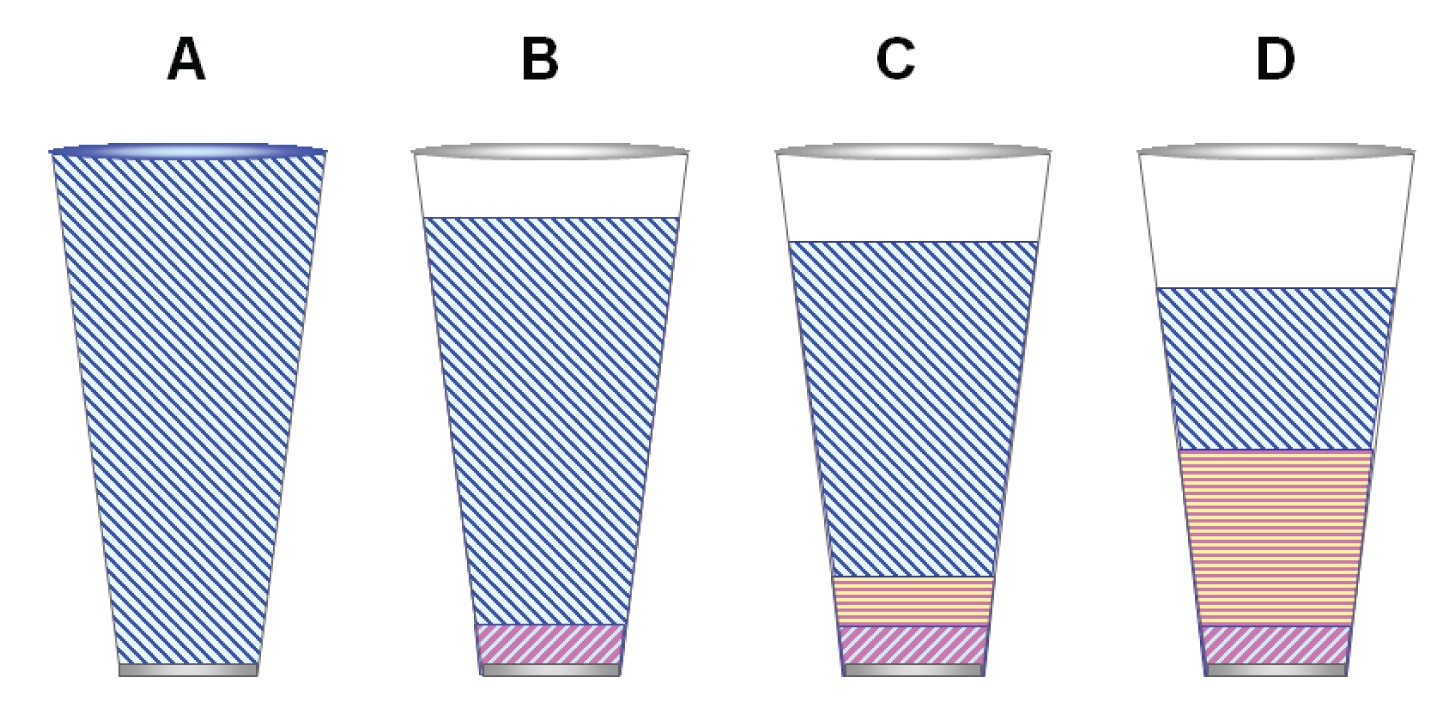
Figure 1: (A) Totally dispersive (apolar) phase like squalane. (B) Apolar silicone stationary phase like 100% polydimethylsilicone contains a small amount of dipole character. (C) Slightly polarizable silicone like 5% phenylmethyl silicone (D) More polarizable phase like 50% phenylmethyl silicone.
Dispersive (apolar) interactions are always present and scale as function of the molecular size of the solute. Polar interactions (hydrogen bonding, dipole-dipole, and dipole-induced dipole) are sometimes present, and range in significance based on the nature of the functional group and the amount of polar functional groups relative to the amount of apolar groups in each polymeric repeat unit.
Selectivity can be described as the preferential retention of solutes based on some specific polar interaction or combination of polar interactions. As a completely apolar stationary phase would elute compounds based on their molecular sizes, it would be considered to have no selectivity. Just to be clear, for selectivity to have any practical meaning in a given separation, the sample mixture must have solutes with diverse chemical structures. If the sample contains only analytes of similar polar functionality (e.g., a homologous series of alcohols) then there is no real difference in separation between using a polar stationary phase or an apolar phase - the alcohols will all elute in order of their molecular size. Their elution temperatures might be different for the same phase ratio of the different stationary phases, but the separation will basically look the same.
Figure 1 shows a simplistic view of stationary phase polarity in the form of glasses filled with different interaction mixtures. As the volume of stationary phase is infinite relative to the volume of a solute with which it would interact, it is best to think of a smaller, local interaction zone that is on the order of millimeters wherein a solute band would partition as it passes through the column. The stationary phase volume associated with this zone is represented by the size of the glass. The glasses are filled with the interaction energies characteristic of the phase.
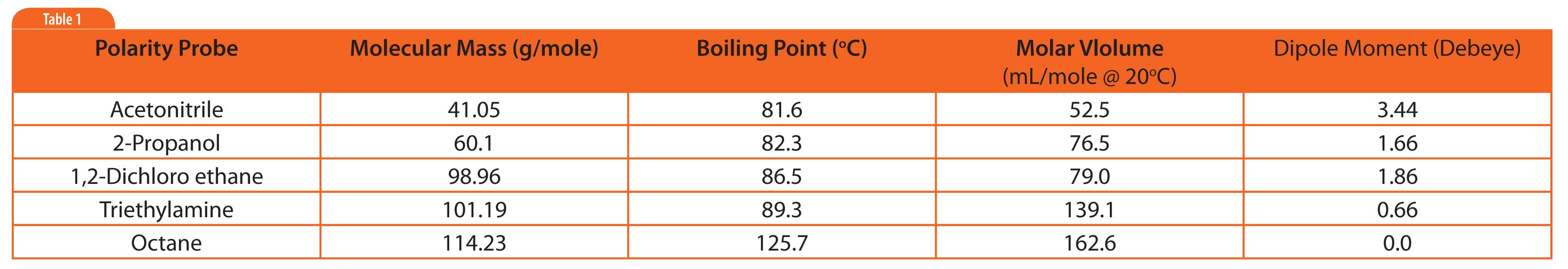
Table 1: Physical properties of polarity probes.
Table 1 lists several probes used to visualize the polarity of stationary phases[1]. They were chosen because they probe dipole and hydrogen bonding character of phases. Octane was included as an apolar reference peak.
There are almost no stationary phases in common use in GC that are purely apolar. The closest is squalane; a high molecule-weight branched hydrocarbon. It has limited use because it cannot be immobilized on the wall of an open tubular column – therefore, it has high bleed and low upper temperature limit. Figure 2(A) shows the elution order of the polarity probes on a squalane packed column. The elution order is in order of increasing molecular weights and boiling points, as expected.
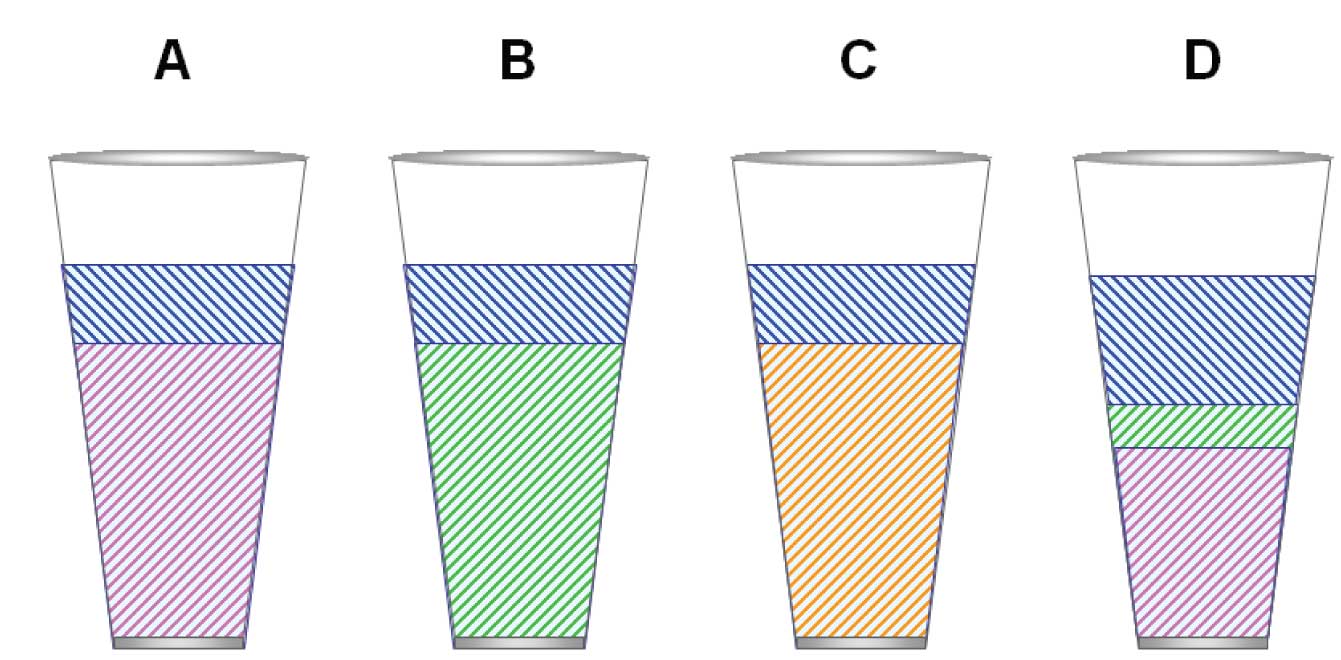
Figure 2: (A) Highly selective dipole phase (e.g., TCEP). (B) Highly selective proton acceptor (basic) phase (hypothetical). (C) Highly selective proton donor (acidic) phase (hypothetical). (D) Realistic polar stationary phase (e.g., carbowax).
Polydimethylsilicone, considered an apolar stationary phase by most chromatographers. Figure 1(A) represents a typical methyl silicone stationary phase such as polydimethylsilicone whose interactions are predominantly dispersive but has a slight dipole character. Figure 3(B) shows that elution of the probes on polydimethylsilicone is the same as with squalane, although overall retention is less. Hence the glass in Figure 1(B) is shown as not being full (less overall interaction than with the primary reference, squalane).
Figures 3(C) and 3(D) show that as the methyl groups are substituted by higher amounts of phenyl groups, polarizability of the phase increases, making the possibility of dipole-induced dipole interactions more significant and at the same time reducing overall retention. In the glass analogy of Figures 1(C) and 1(D), this is reflected by the increased size of polarizable portion relative to the dispersive portion, and decrease in overall volume.
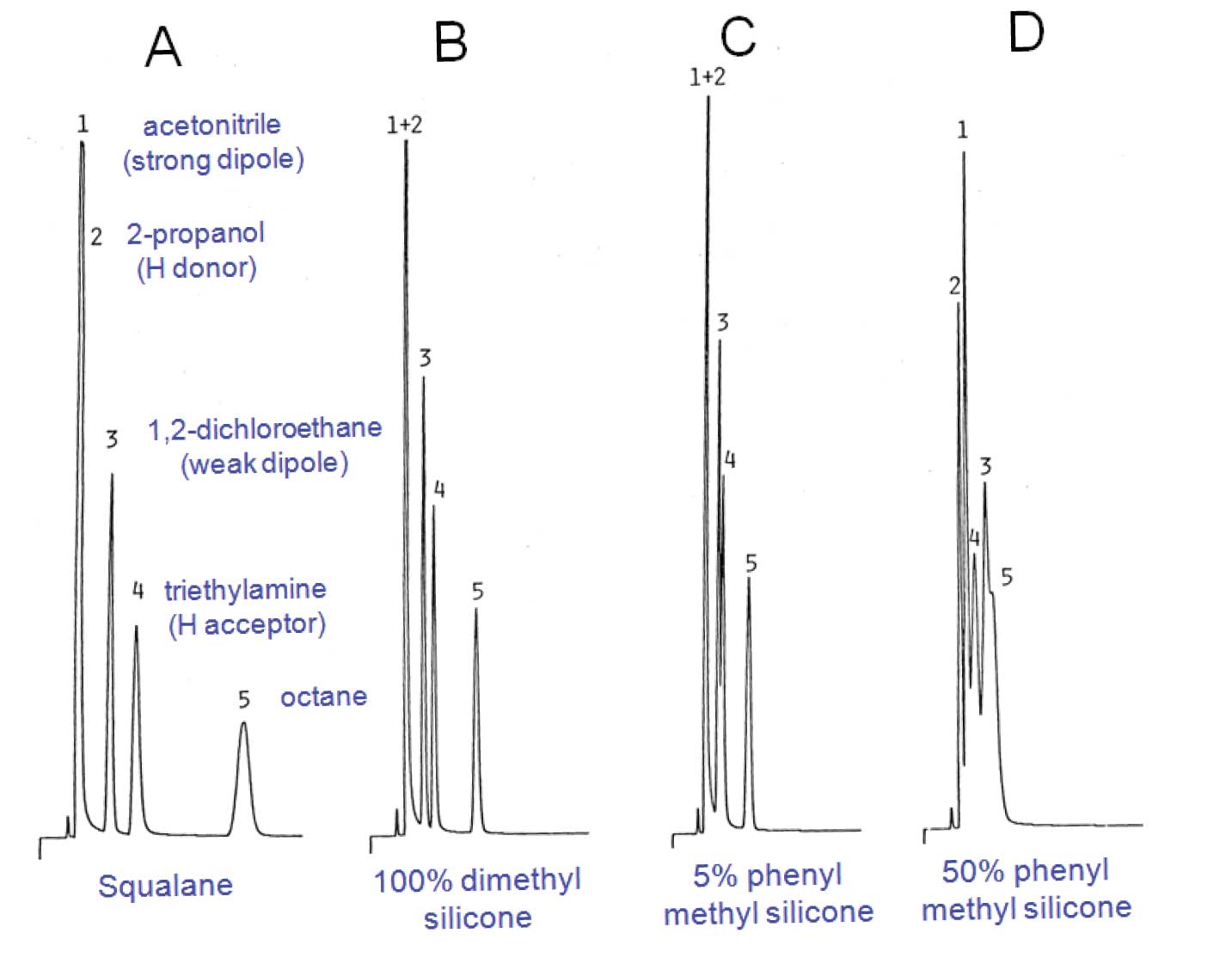
Figure 3: Retention of similar-sized polarity probes on packed columns of identical loadings of different stationary phases. Squalane is a saturated hydrocarbon that has only dispersive (non-polar) interactions polydimethyl silicone, elutes probes in order of molecular size. More dipole-dipole interaction is apparent with higher phenyl content by higher relative retention of dipole probes.
Figure 2(A) illustrates a stationary phase that has a dominant dipole character. Notice that the glass is even less full. Also notice that the amount of dispersive potential is much reduced. This means that compounds with no dipole character will not be retained much at all.
This blog article series is produced in collaboration with Dr Matthew S. Klee, internationally recognized for contributions to the theory and practice of gas chromatography. His experience in chemical, pharmaceutical and instrument companies spans over 30 years. During this time, Dr Klee’s work has focused on elucidation and practical demonstration of the many processes involved with GC analysis, with the ultimate goal of improving the ease of use of GC systems, ruggedness of methods and overall quality of results. If you have any questions about this article send them to techtips@sepscience.com




