To fully grasp the concepts of retention and selectivity of GC stationary phases, one must first understand the fundamental intermolecular interactions that lead to retention. In the second part of this series we discuss polar interactions; the interactions responsible for any observed selectivity in GC.
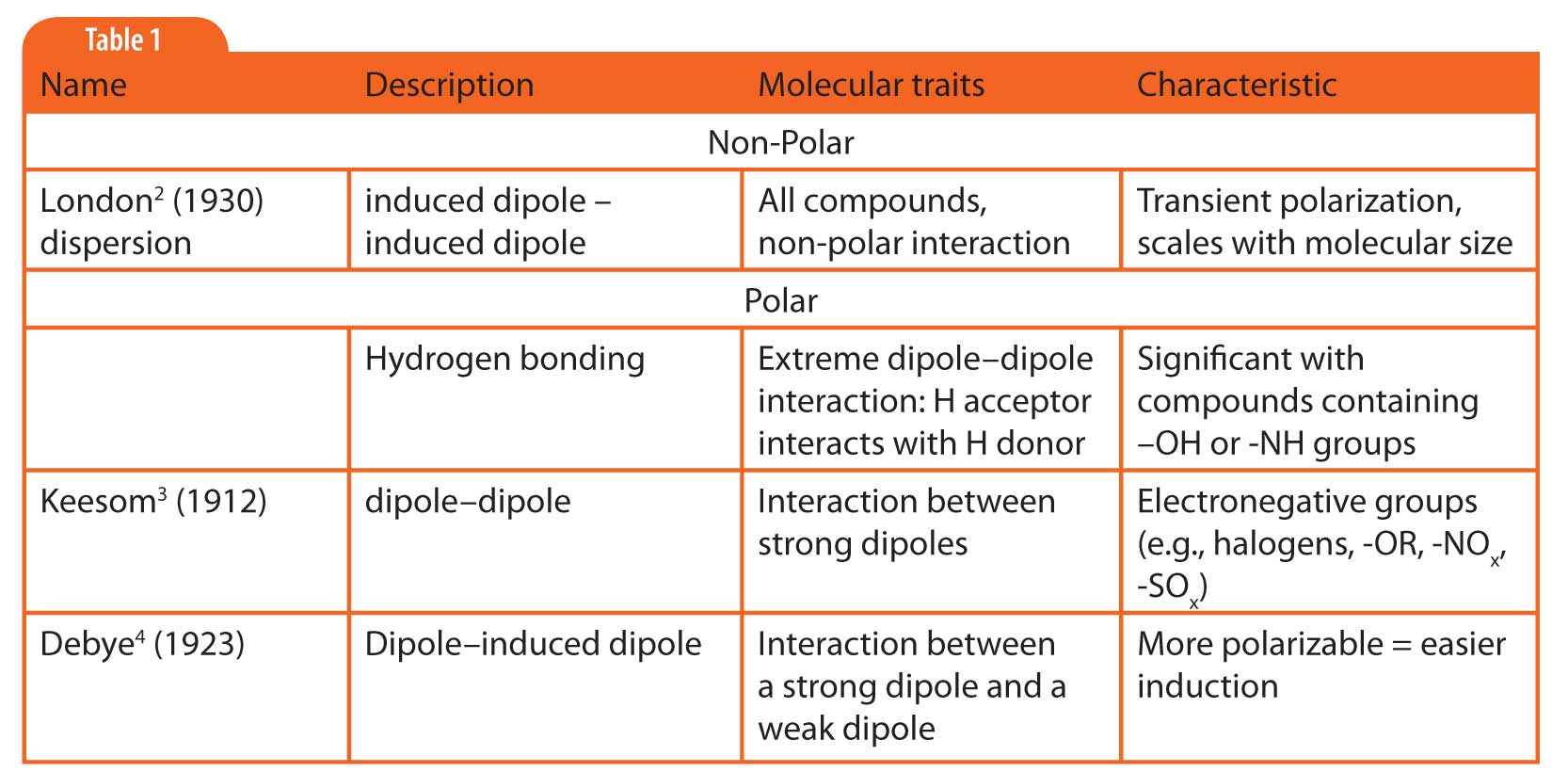 Table 1: Van der Waals forces of interactions between molecules.
Table 1: Van der Waals forces of interactions between molecules.
Retention in gas chromatography (GC) is related to the strength of interaction between the solute molecules and the stationary phase. There are only a few available intermolecular forces of importance in gas chromatography. For reference they are listed in Table 1.
The strength of interaction between a solute and a stationary phase is a function of the total of all possible non-polar and polar interactions. As discussed last month, all molecules can interact to some degree through non-polar, dispersive interactions. However, if polar functional groups are present in either or both the solute or stationary phase, then polar interactions can contribute to the retention.
Polar selectivity arises from the different strengths and types of polar interactions between stationary phase and sample components. Polarity is a function of the nature of the polar groups as well as how they are structured in the molecule (proportion to non-polar groups, shielding, interactions with neighboring groups, etc.). When polar interactions between a solute and a stationary phase are significant, that solute is preferentially retained relative to other compounds of similar molecular weight.
All three of the possible classes of polar interactions in gas chromatography arise from permanent distortions in the electron orbitals of at least one of the interacting molecules (solute or stationary phase). Attraction between molecules resulting from these distortions leads to stronger retention. The strength of these distortions and the susceptibility of other molecules to them are what differentiate the three classes of polar interactions.
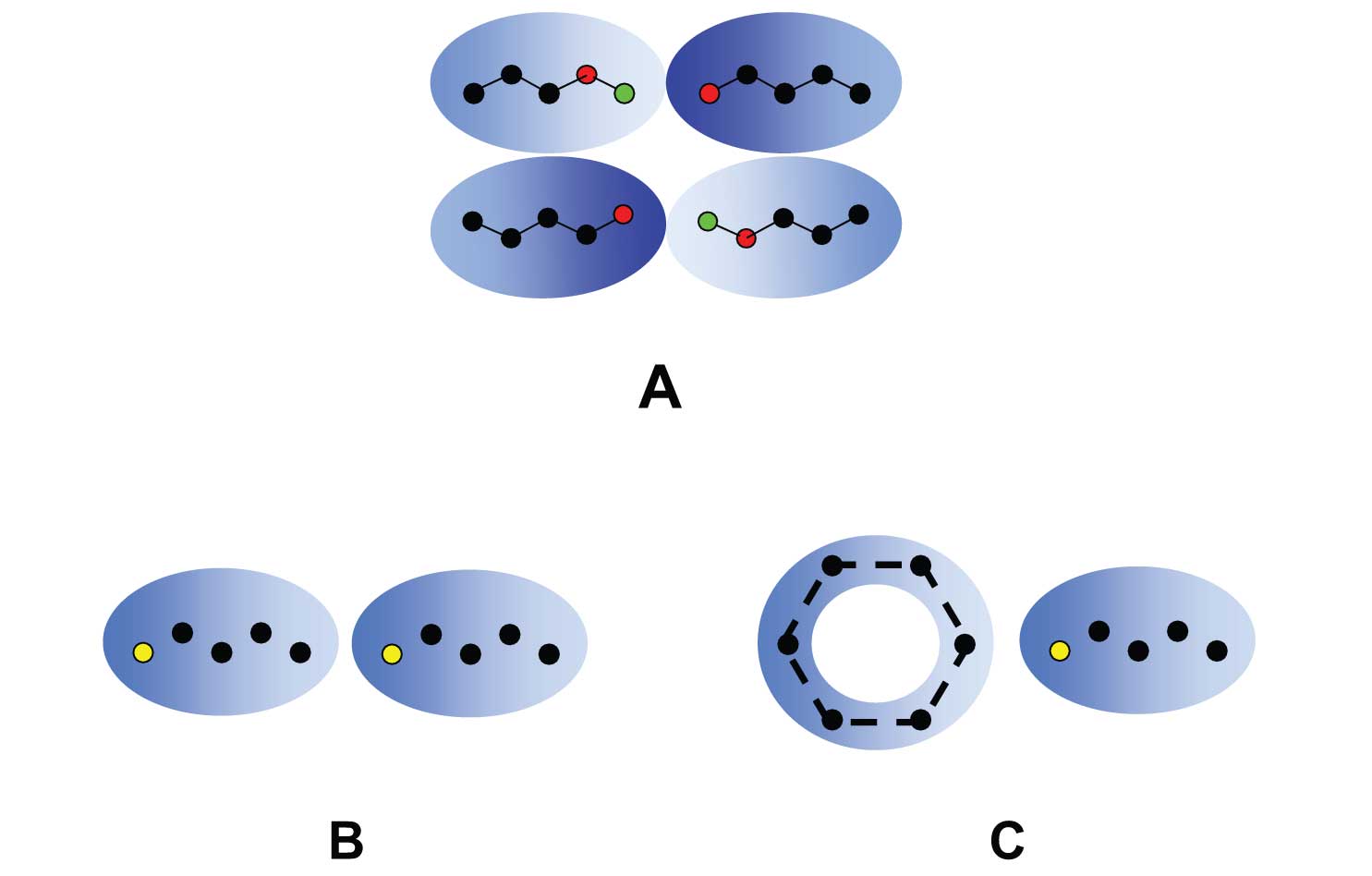 Figure 1: Three polar interactions important in gas chromatography. (A) Hydrogen bonding requires both a hydrogen donor (acidic proton, H; green dots) and a hydrogen acceptor sites (basic, electron rich, N, O; red dots). The blue cloud is shaded to illustrate electron density (darker = electron rich, lighter = electron poor). (B) Dipole-dipole interactions occur between molecules that have a permanent dipole due to electronegative atom in the molecule (yellow dot). (C) Dipole-induced dipole happens when a dipole distorts the electron cloud of a polarizable molecule such as an aromatic, causing a momentary dipole.
Figure 1: Three polar interactions important in gas chromatography. (A) Hydrogen bonding requires both a hydrogen donor (acidic proton, H; green dots) and a hydrogen acceptor sites (basic, electron rich, N, O; red dots). The blue cloud is shaded to illustrate electron density (darker = electron rich, lighter = electron poor). (B) Dipole-dipole interactions occur between molecules that have a permanent dipole due to electronegative atom in the molecule (yellow dot). (C) Dipole-induced dipole happens when a dipole distorts the electron cloud of a polarizable molecule such as an aromatic, causing a momentary dipole.
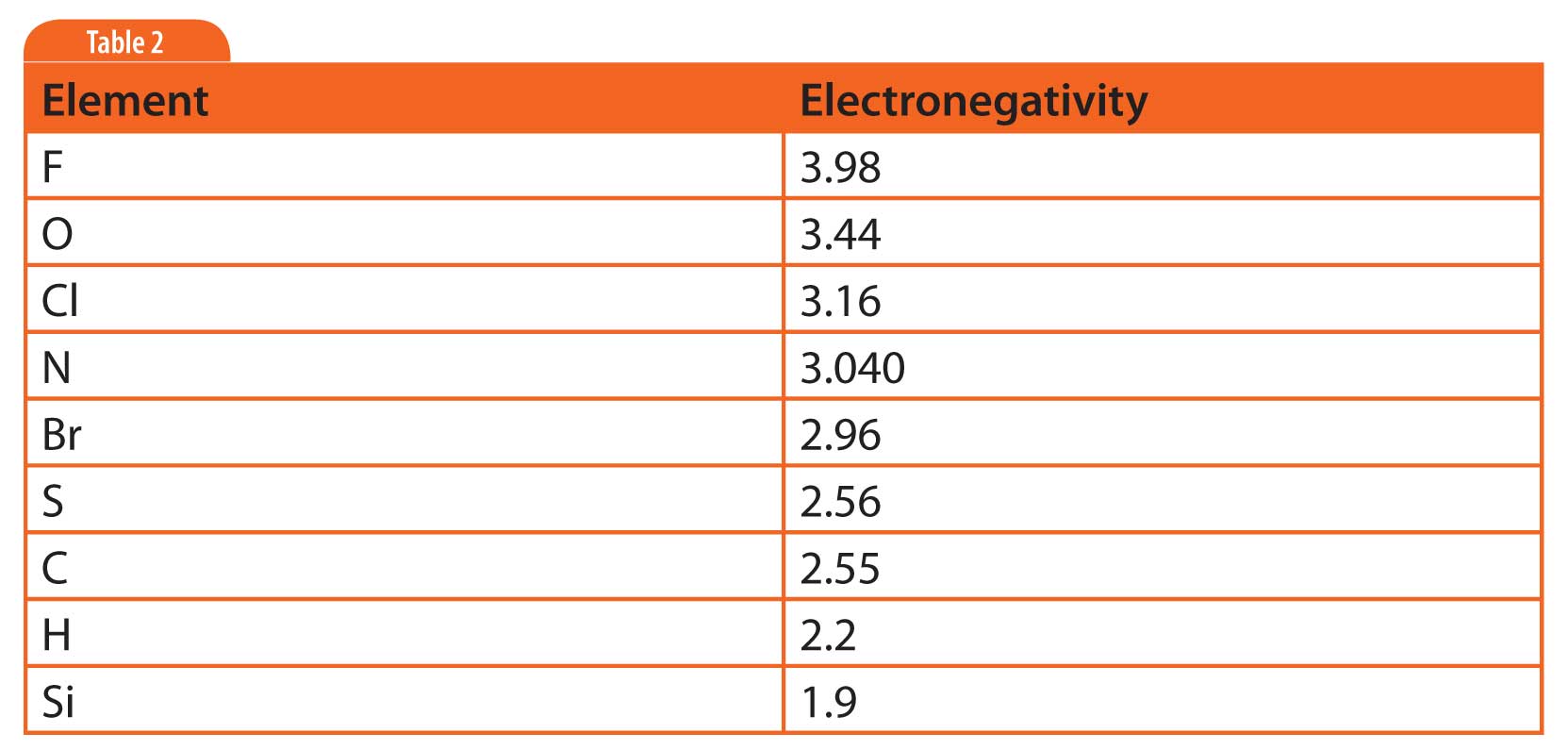
The distributions of electrons in orbit around molecules are often described as “electronic clouds”. Distortions in the clouds happen when there is an imbalance in the relative electron affinity of atoms bound in the molecule. A measure of an atom’s electron affinity is its electronegativity . Atoms toward the top and right of the periodic table of the elements tend to be the most electronegative. Table 2 lists the electronegativity of elements common to compounds analysed by GC.
Electronegative atoms in a molecule attract electrons, distorting the electron cloud around the molecule and causing a slight electronegative bias (δ-) to the molecule (Figure 1). The bias is considered permanent because the distortion is a function of the molecular structure and is always present (as opposed to the transient distortions that were described in last month’s discussion of non-polar forces of interaction).
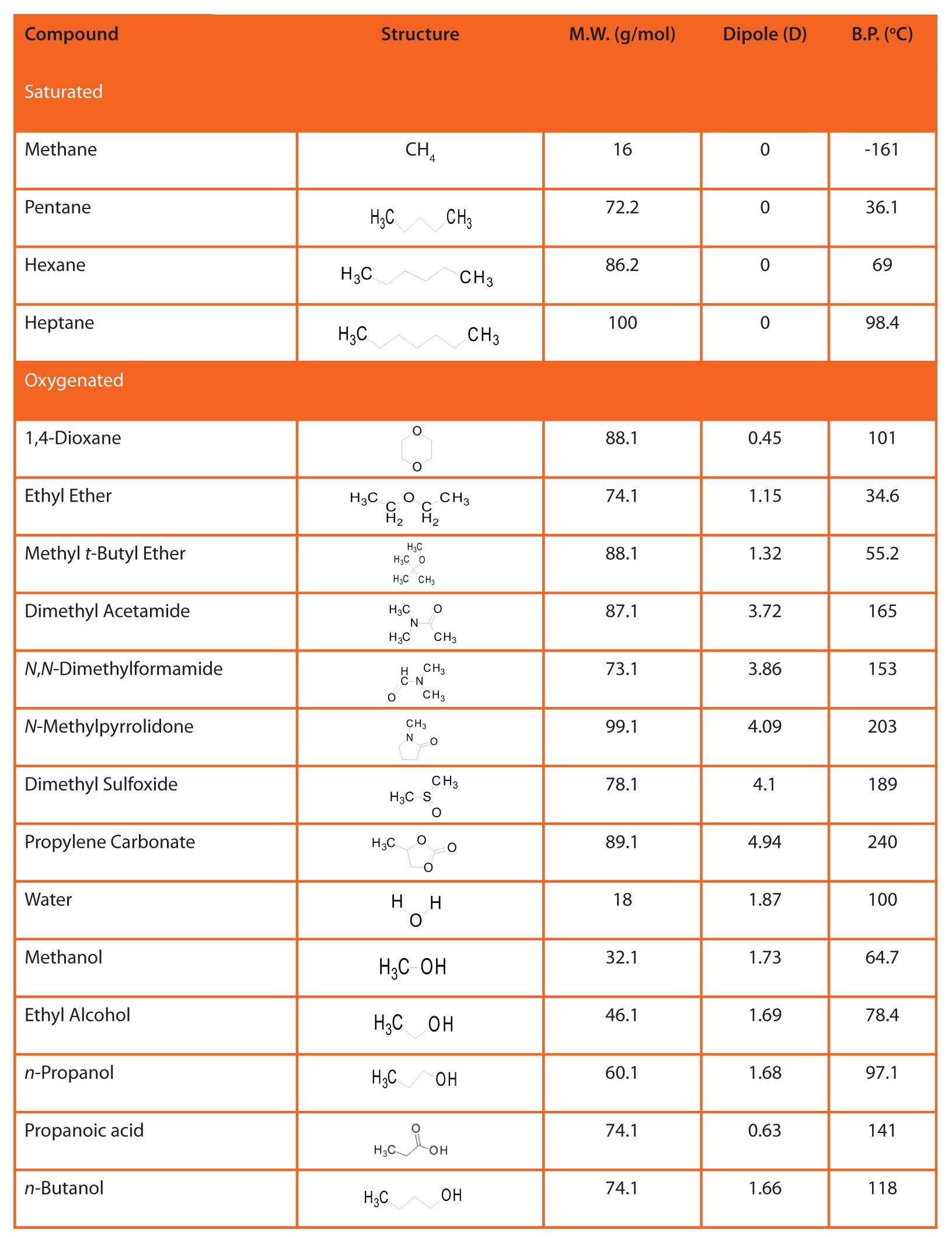 Table 3: Physical properties of some volatile organic compounds.
Table 3: Physical properties of some volatile organic compounds.
A molecule with such a sustained distortion in the electron cloud is said to have a permanent dipole . A measure of the strength of this dipole in a given molecule is its dipole moment. Table 3 lists the dipole moments of several light molecules. The position of electronegative atoms relative to others in the molecule, resonance possibilities, symmetry, etc. can influence the magnitude of the dipole moment.
When two permanent dipoles near each other, oppositely biased ends align (opposites attract, likes repel), as illustrated in Figure 1(B). This result is called a dipole-dipole interaction because both molecules have permanent dipoles. The stronger the dipole moments of either or both of the stationary phase or solute, the stronger the interaction and the higher the retention.
Hydrogen bonding is an extreme case of dipole-dipole interaction , where the intermolecular interactions are very strong [Figure 1(A)]. The highly electronegative atoms nitrogen (N) and oxygen (O) are especially electron rich when present in organic molecules [note: even though very electronegative, F bound to organic compounds does not significantly hydrogen bond]. These heteroatoms act as strong electron donors or, in other words strong proton (hydrogen) acceptors. If a hydrogen (H, proton) atom is bound to either O or N in a molecule (e.g., in alcohols or amines), its lone electron is easily attracted away by the highly electronegative atom, rendering it highly electron deficient. This proton then becomes a strong electron acceptor. In other words, it becomes a strong proton donor.
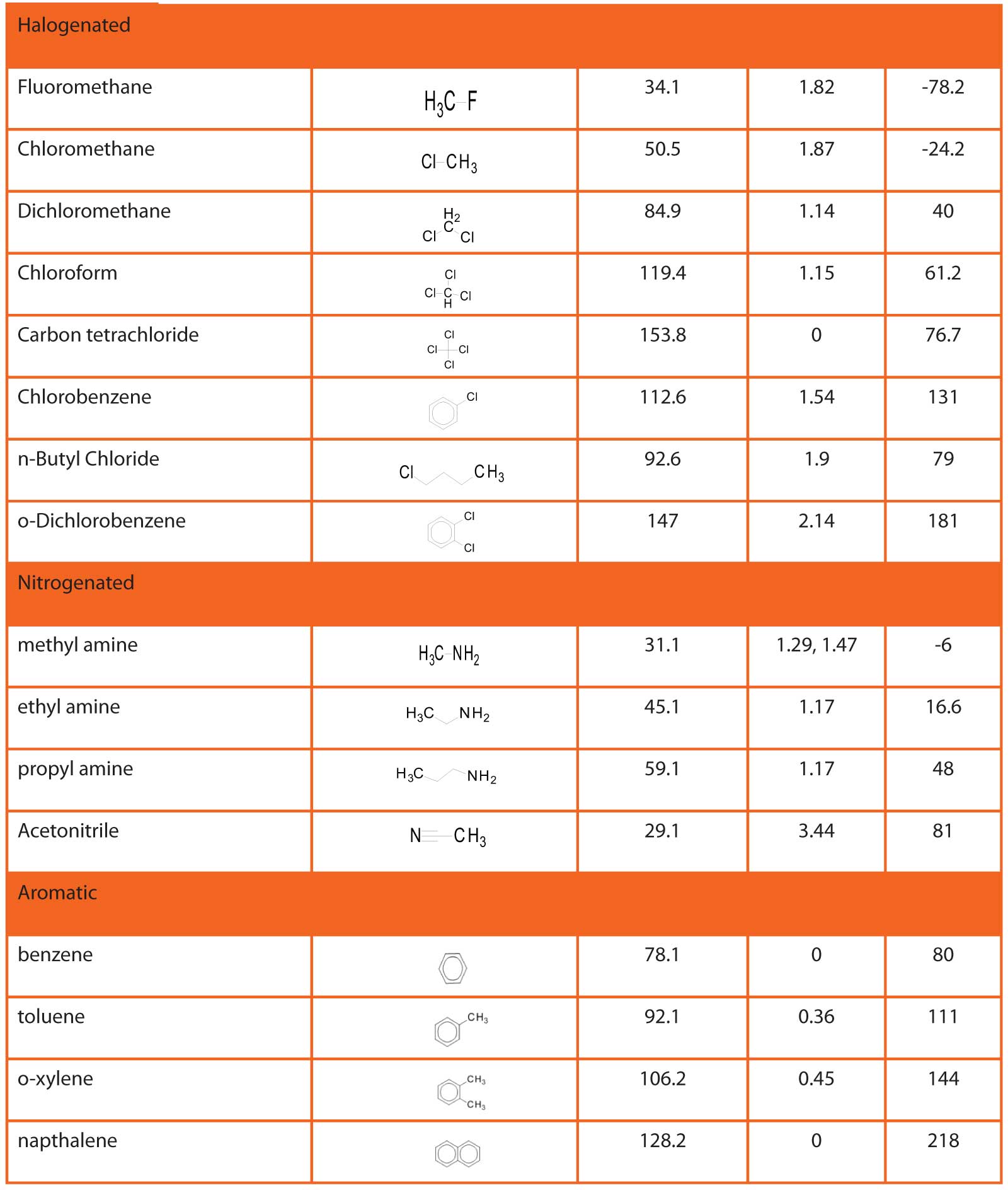
Table 4: Physical properties of some volatile organic compounds.
This blog article series is produced in collaboration with Dr Matthew S. Klee, internationally recognized for contributions to the theory and practice of gas chromatography. His experience in chemical, pharmaceutical and instrument companies spans over 30 years. During this time, Dr Klee’s work has focused on elucidation and practical demonstration of the many processes involved with GC analysis, with the ultimate goal of improving the ease of use of GC systems, ruggedness of methods and overall quality of results. If you have any questions about this article send them to techtips@sepscience.com




