In the previous two articles ('#7 Fundamental Resolution Equation, Part 1' and '#8 Fundamental Resolution Equation, N'), we’ve looked at the fundamental resolution equation
Rs = ¼ (N)0.5 (a-1) (k / [1+k]) (1)
Where N is the column plate number or efficiency, α is the selectivity, and k is the retention factor. In using equation 1 as a method development guide, we saw that the first step is to choose a column with a plate number that is likely to separate the complexity of sample we have. Often a N ≈ 10,000 is sufficient for our needs. This can be obtained with a 150 x 4.6 mm, 5 µm or 100 x 4.6 mm, 3 µm column operated at 1-2 mL/min for reasonable run times. Now let’s move to the right in equation 1 and look at the separation factor, α.
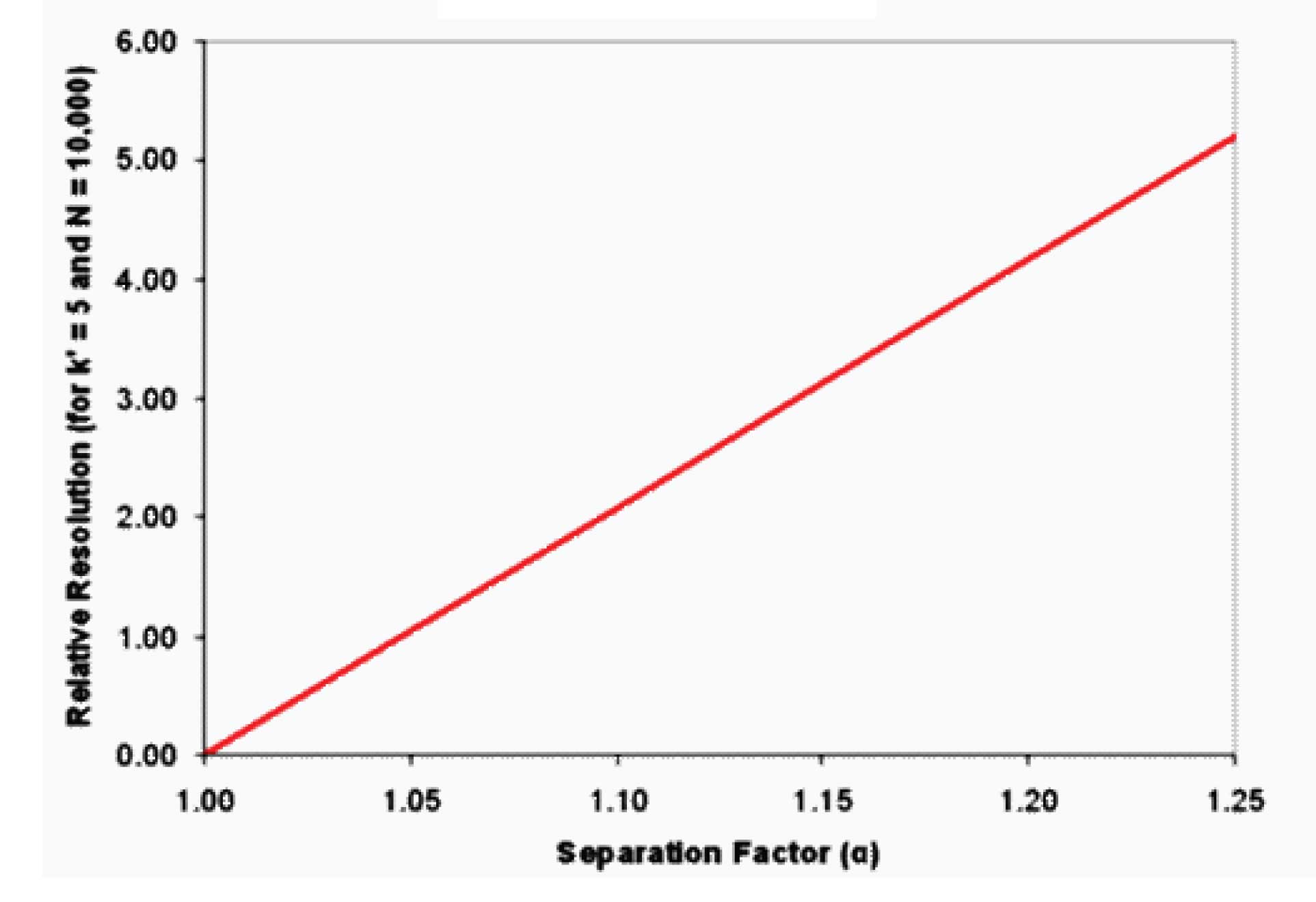 Figure 1
Figure 1
In a similar way to what we did in the previous article (#8 Fundamental Resolution Equation, N) for N, if we plot Rs vs. a, we see the relationship shown in Figure 1. This is a very unexciting graph, but what it tells us is that as we increase a, we get a direct increase in Rs. This catches my attention – if I double alpha, I double resolution. That’s a much better return than doubling the plate number only to receive an optimistic 40% increase in resolution.
In an earlier discussion of α (Back-to-Basics #3: Selectivity) we saw that a is changed by making chemical changes in the system – in the mobile phase, stationary phase, or sample. A change in mobile phase pH is the most powerful way to change α, but it only works for ionizable compounds. A more universal way to change α is to change the mobile phase solvent – acetonitrile (ACN) to methanol (MeOH) or tetrahydrofuran (THF) are the most common choices. Another option is to change the column chemistry, but the column labels of C18, CN, or AQ aren’t necessarily useful in this regard. In the very first HPLC Solutions (#1) , a database for comparing column selectivity was introduced – this database can be used to help find columns that have different selectivity from each other. To a lesser extent, a change in the solvent strength (%-organic solvent), gradient time, or temperature can be used to change α.
Although a change in a is the most powerful way to change resolution, because of the relationship shown in Figure 1, it is not very predictable without prior knowledge. That is, I’m willing to bet that the chromatogram will look different if I change the mobile phase organic solvent from ACN to MeOH, but I can’t tell you if the separation will get better or worse. And although an overall change in selectivity for the chromatogram is likely with such a change, for a given peak pair, the separation may increase, decrease, or not change at all. Because of the unpredictability of changing a without prior knowledge of the system chemistry, improving the separation by changing α is best left until other variables have been explored – specifically a change in the retention factor, k.
This blog article series is produced in collaboration with John Dolan, best known as one of the world’s foremost HPLC troubleshooting authorities. He is also known for his research with Lloyd Snyder, which resulted in more than 100 technical publications and three books. If you have any questions about this article send them to TechTips@sepscience.com




