Q: I have developed a method based on an article from literature for testing an analyte along with its impurities and degradants. This is a gradient method that utilizes a C18 column with 0.1% trifluoroacetic acid (TFA) in water (A) and 0.1% TFA in 80% acetonitrile/20% methanol (B). I tried other mobile phase additives to buffer the system (acetic acid, phosphoric acid, sodium phosphate) but using TFA resulted in the best peak shape and resolution. I’m not sure if that is the result of silanol suppression or ion pairing or both. However, I am concerned about (1) the stability of my column and (2) the stability of the TFA itself in the mobile phase. I’ve seen some baseline disruptions near the retention time of the analyte that ordinarily would be insignificant, but because I’m trying to show that the method can detect related substances down to 0.1% then it does become an issue with peaks near the lower limit of quantification, LLOQ. The issue with the baseline was not reproducible…it went away when I made a new batch of mobile phase. Do you have any recommendations for (1) extending the column life using TFA, and (2) minimizing baseline issues resulting from TFA degradation?
A: First, you are not using conditions that are likely to damage the column. This recipe for using 0.1% TFA is widespread and should cause no ill effects. The pH of 0.1% TFA is approximately 2, and today’s high-purity silica (type-B) columns are stable in the 2 < pH < 8 range, so you should have no worries there. Also, it is likely that it is TFA’s unique combination of low pH and good ion-pairing properties that make it work in the present case, where other buffers fail.
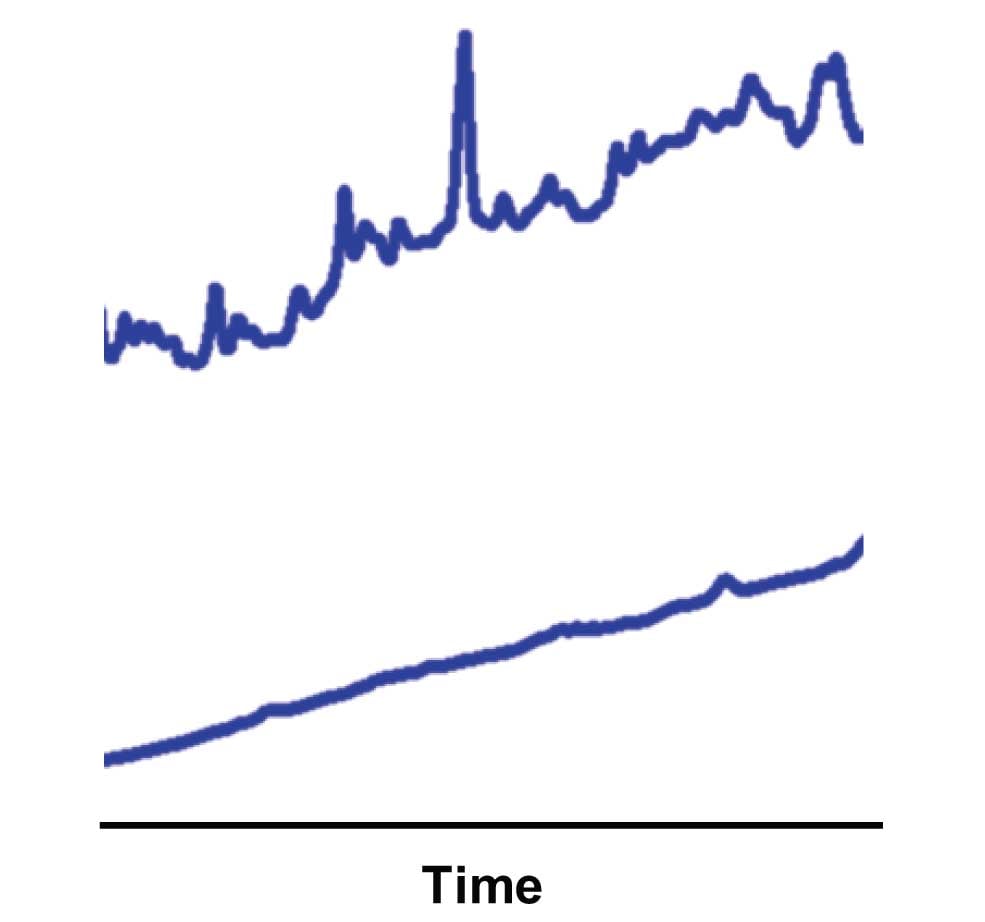 Figure 1
Figure 1
So what about the issue of baseline stability? Unfortunately, TFA is not very stable in its pure form once exposed to the air. I recommend purchasing HPLC-grade TFA in 1-mL ampoules for a single-use strategy. If you look in the chemical catalogue, you’ll see that you can buy a 25- or 100-mL bottle for about the same price as ten 1-mL ampoules, and it is a tempting way to save money, but I don’t advise this route. In my experience, the larger containers are good only for one use, too. For example, I have tried opening the container in a glove bag in an inert atmosphere, sealing the bottle with wax after use, and other attempts to preserve it. But each time the bottle was opened a second time, it was contaminated, as shown by extra peaks appearing in a blank gradient. So play it safe and buy your TFA in ampoules.
As far as stability of TFA in mobile phase goes, I don’t think there should be much of a problem there. Although TFA is quite volatile as a neat solution, once it is in water, it will ionize and should not evaporate. However, all mobile phases age, and your problem merely may be one of trying to use the mobile phase for too long a time. For example, Figure 1 shows the difference between blank gradient baselines for a freshly prepared buffer (lower) and one that has become contaminated over time (upper). I recommend replacing the aqueous mobile phase once a week. Many workers extend this to two weeks without problems, but again, I say why take the risk? Organics should be good for a month.
Be sure to replace the reservoirs with clean ones when you make a new batch of mobile phase – you don’t want to contaminate the fresh batch with a dirty container. And if you suspect that the system is contaminated, as might be the case if the aqueous mobile phase was used for longer than two weeks, it would be wise to replace the sinker frit in the reservoir – this could be another source of cross-contamination.
This blog article series is produced in collaboration with John Dolan, best known as one of the world’s foremost HPLC troubleshooting authorities. He is also known for his research with Lloyd Snyder, which resulted in more than 100 technical publications and three books. If you have any questions about this article send them to TechTips@sepscience.com




